Developmental and Reproductive Toxicology (DART) Studies: How Do They Fit into Your Program?
Development and reproductive toxicology studies, or DART studies, are required for most non-oncology programs between IND and NDA filings. Their goal is to detect any effects of a drug within a complete reproductive cycle as relevant to humans — from initial conception to reproductive capacity in the next generation. They can be designed to measure the effects of a drug on male and female fertility, the full span of embryonic and fetal development (EFD), and pre- and post-natal development (PPND), including gestation, parturition, lactation, and teratology. In addition, juvenile studies performed on an as-needed basis can look at a drug’s impact on neonates to adolescents.
Timing of DART studies
DART studies complement the standard adult nonclinical toxicology and safety testing required for all drug approvals, and they can be similarly tailored to each program. In general, they run parallel to clinical trials depending on the target populations, typically starting around Phase 1 and finishing prior to the end of Phase 3. Ideally, a sponsor would have kinetics data available (though not necessarily in pregnant animals) prior to the start of these studies.
DART studies by segment
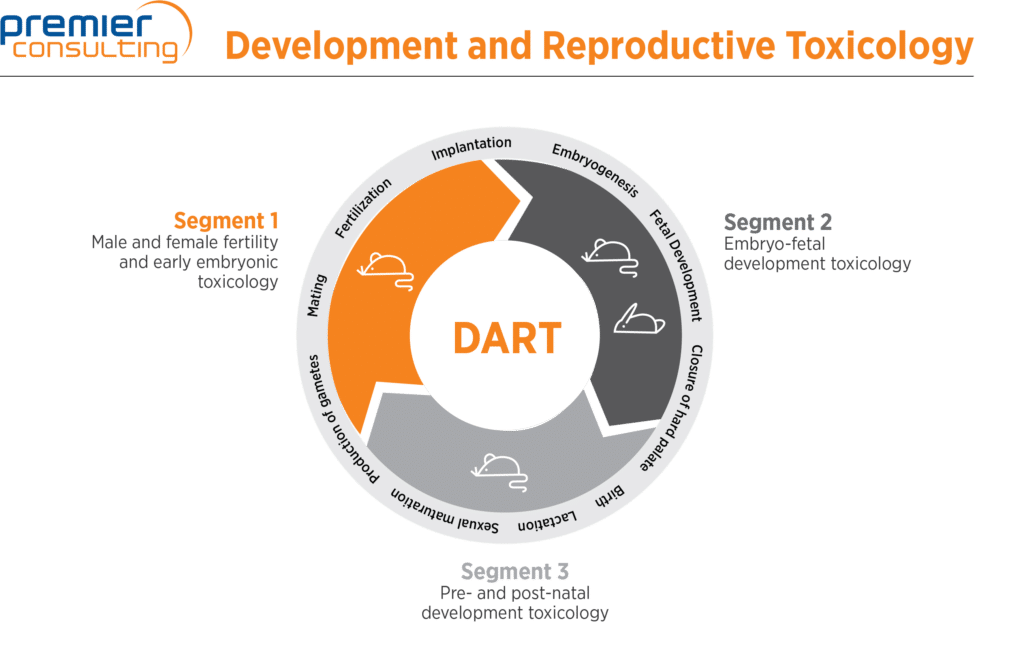
DART studies are categorized in three segments based on the timing of the test item administration in the reproductive cycle and the ensuing observed effects. A fourth category consists of the juvenile studies, which focus on a drug’s effects on the pediatric population.
| Category | Name | Administration and Observation Timing | Target for Observation | Typical Species |
| Segment I | Fertility and embryonic development | From production of gamete to mating for males, and through implantation for females | Estrous cyclicity, spermatogenesis, mating behavior, fertilization, early embryogenesis | Rodent (rat or mouse, both sexes) |
| Segment II | EFD | From implantation to closure of hard palate | Late embryogenesis, early fetal development including major organ formation | Rodent (female rat or mouse), non-rodent (female rabbit) |
| Segment III | PPND | From closure of hard palate to weaning (observation includes second generation) | Late fetal development, parturition, lactation, weaning, offspring growth, maturation including second generation | Rodent (rat or mouse, both sexes) |
| N/A | Juvenile | From neonate stage to adolescence | Pediatric population | As relevant |
Considerations for DART studies
Timing: GLP regulations require that most drug development programs include at least one study per segment. However, exceptions are permitted, and the timing can be variable. For example, sometimes preliminary EFD studies are all that is required to allow a limited inclusion of women of childbearing potential in clinical trials, and executing a complete toxicology program can be delayed.
Optimization: As the name indicates, DART studies must be designed to observe and document both developmental (mortality, structural, and functional impairments, and maturation delays) and reproductive (male and female fertility, parturition, and lactation) toxicities. A sponsor can optimize a DART program to reduce the required number of animals and streamline timelines and budgets through several methods, such as including multigenerational study designs that combine the various study types or considering qualified alternative assays.
CMC planning: Test item requirements are particularly difficult to predict, as they are heavily dependent on pregnancy rates and litter sizes and on study designs and the animal numbers required to reach statistical significance. In conjunction with a sponsor’s chosen CRO, our teams can support DART CMC planning by interpreting recent species and program-specific data in the context of approved study designs.
Species selection: The preferred species for DART studies are often rats as the rodent, and rabbits as the additional non-rodent, for EFD studies. This is mostly due to practical considerations, the availability of historical data, and the length of the reproductive cycles. Other species are available, but all have disadvantages: sensitivity to sexual hormones for rats, difficulty in interpreting clinical signs for rabbits, long fetal periods for guinea pigs, and stress sensitivity and small fetuses for mice, to name a few. Details can be found in this FDA guidance.
Biologics: Several factors should be considered for biologic products such as monoclonal antibodies, for which NHPs are often the only relevant species. Relevant concerns include the feasibility of observing the offspring through maturity and into a second generation, and whether it is possible to replace a fertility assessment with a thorough histopathology assessment of the reproductive tissues in earlier chronic repeat-dose studies.
Overall, designing and executing development and reproductive studies can be a complex undertaking, due to the expert knowledge required to fully optimize their designs. The experienced team at Premier Consulting can partner with you to optimize your DART program and develop a risk reduction strategy.