What You Need to Know About ICH Q14 and ICH Q2(R2), Part 1
For researchers who are developing or working with analytical procedures, the new ICH Q14 Analytical Procedure Development and revised ICH Q2(R2) Validation of Analytical Procedures drafts represent foundational guidance documents. Together, they describe suggested development and validation activities for the lifecycle of an analytical procedure used to assess the quality of drug substances and drug products. ICH Q14 describes scientific principles and risk-based approaches for developing and maintaining suitable analytical procedures, while ICH Q2(R2) provides guidelines for establishing, submitting, and maintaining evidence that an analytical procedure is fit for purpose (assuring drug quality).
The target date for finalization and implementation of these documents in the local regional regulatory system is May 2023; this two-part blog series explains everything you need to know to prepare. (Read Part 2 here.)
Overview of ICH Q14
ICH Q14 applies to both new and revised analytical procedures used for release and stability testing of clinical through commercial drug substances and products. It can also be applied to analytical procedures used as part of a drug product control strategy. Importantly, ICH Q14 emphasizes that the scientific principles within it can be applied in a phase-appropriate manner during the product’s lifecycle and are not intended to introduce new regulatory requirements.
Table 1. Descriptions of Key Sections of ICH Q14
| Section | Description |
| Introduction | Describes the objective of the guideline |
| Scope | Outlines how the guideline should be applied and details the analytical procedure lifecycle (see Figure 1) |
| Analytical Target Profile (ATP) | Defines the ATP |
| Knowledge and Risk Management in Analytical Procedure Development and
Continual Improvement |
Describes how knowledge should be used and managed and encourages the use of quality risk management |
| Evaluation of Robustness and Parameter Ranges of Analytical Procedures | Defines robustness and the data needed to support parameter ranges |
| Analytical Procedure Control Strategy | Provides guidance on ensuring that the analytical procedure performs as expected during routine use throughout its lifecycle according to Established Conditions (ECs) |
| Lifecycle Management and Post-Approval Changes of Analytical Procedures | Outlines how to manage and report modifications to the analytical procedure |
| Development of Multivariate Analytical Procedures | Describes considerations and expectations for important aspects of the development of multivariate analytical procedures |
| Development of Analytical Procedures for Real Time Release Testing: Special
Considerations |
Provides a definition and requirements for real time release testing |
| Submission of Analytical Procedure Related Information | Offers guidance on where and what analytical procedure-related information should be included in the Common Technical Document (CTD) |
Figure 1. The Analytical Procedure Lifecycle
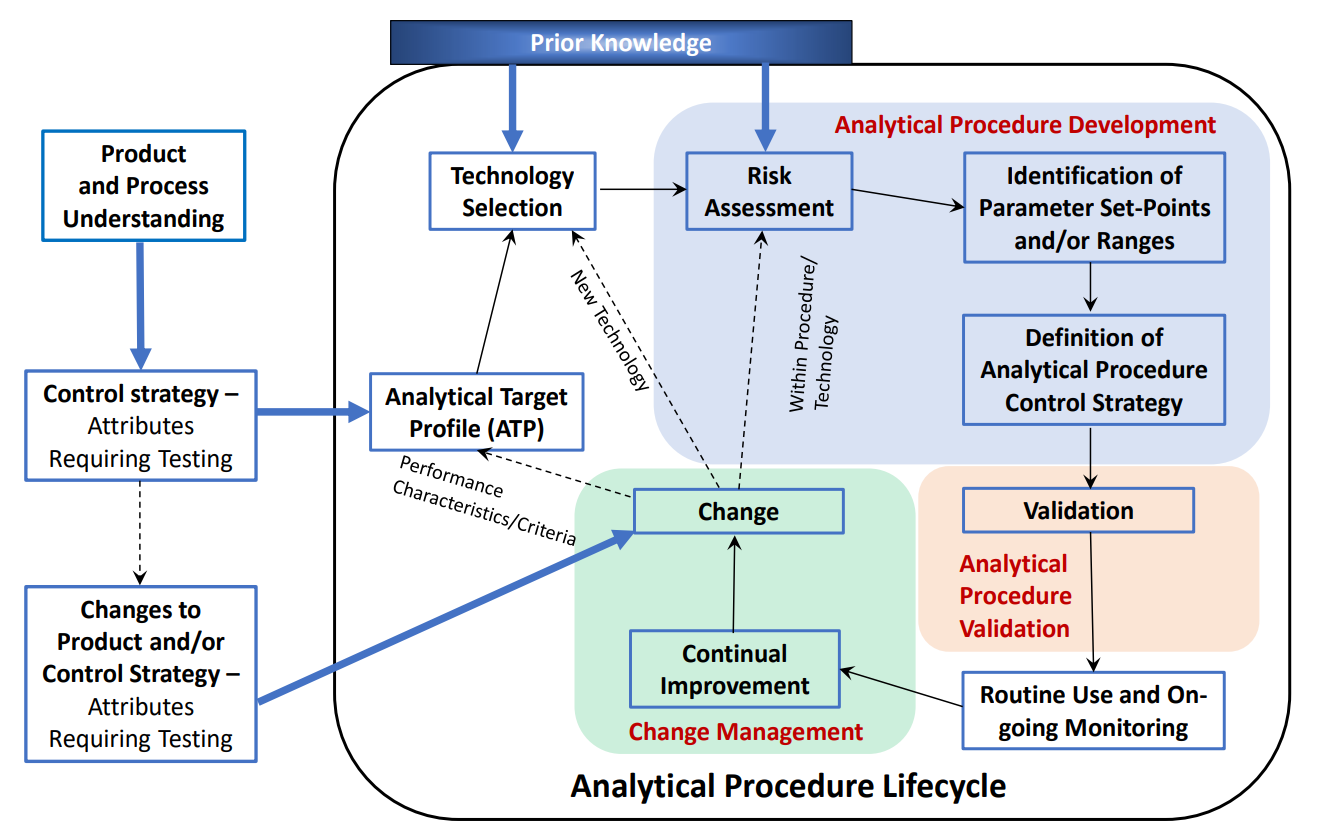
Source: ICH Q14
Introduction of an Enhanced Approach to Analytical Procedure Development
The guideline discusses both a minimal, or traditional, approach and elements of an enhanced approach for analytical procedure development (see Table 2). The enhanced approach integrates elements of quality by design (QbD) and risk management into the development of analytical procedures. This reflects a paradigm shift introduced in ICH Q8, Q9, and Q10, which focus on understanding the process, maintaining a state of control, and pursuing continuous improvement.
A key element of the enhanced approach is the analytical target profile (ATP), a summary of the expected characteristics of the analytical procedure. While application of the enhanced approach is not mandatory, applying individual elements of the approach to analytical procedure development is encouraged. In addition to describing the intended purpose of the procedure, the ATP outlines anticipated performance criteria and Critical Quality Attributes (CQAs), which facilitate technology selection, procedure design and development, performance monitoring, and continual improvement. As with other types of target profiles used in QbD-driven drug development, the ATP may evolve over the lifecycle of the procedure and can be used a as a basis for lifecycle management.
Table 2. Comparison of Minimal and Enhanced Approaches to Analytical Procedure Development
| Minimal Approach | Enhanced Approach |
| Testing identifying attributes of the procedure | Evaluating sample properties |
| Selecting appropriate technology and related instruments | Defining the analytical target profile (ATP) |
| Conducting appropriate development studies | Conducting a risk assessment and evaluating prior knowledge |
| Defining the description of the procedure | Conducting uni- or multi-variate experiments |
| Defining an analytical procedure control strategy | |
| Defining a lifecycle change management plan |
ICH Q14 also:
- Describes important considerations in the development of multivariate analytic procedures and real time release testing
- Provides principles to support change management of analytical procedures
- Explores factors related to the submission of analytical procedure development and related lifecycle information in the CTD format
ICH Q14 is designed to harmonize scientific approaches and terminology for analytical procedure development, streamline the analytical procedure lifecycle, and facilitate continual improvement. Using predefined performance characteristics guides development and facilitates change management. Applying the principles described in the guideline can improve communication between manufacturers and regulators and lead to more efficient approval and post-approval change management.
Part 2 of this series offers an overview of ICH Q2(R2). Contact us to learn how we can support you in applying these guidance documents to your development program!
Authors:
Constance Morris
Manager, Regulatory Affairs CMC
Ankita Rohishaliya
Manager, Regulatory Affairs CMC
References:
- International Council for Harmonisation. ICH Harmonised Guideline: Analytical Procedure Development Q14. Available at https://database.ich.org/sites/default/files/ICH_Q14_Document_Step2_Guideline_2022_0324.pdf.
- International Council for Harmonisation. ICH Harmonised Guideline: Validation of Analytical Procedures Q2(R2). Available at https://database.ich.org/sites/default/files/ICH_Q2-R2_Document_Step2_Guideline_2022_0324.pdf.