The Opportunity for Generics Companies and Value Added Medicines in the US and Europe
To succeed in today’s highly competitive pharmaceutical landscape, differentiation is key. In recent years, many generics companies have turned their focus to niche products, complex generics, or biosimilars to achieve this differentiation.
During the 3rd Annual Portfolio Management Strategies for Biosimilars and Generics in Barcelona last month, Premier Consulting shared insights on an increasingly common way to gain differentiation: Value Added Medicines.
What are Value Added Medicines?
Value Added Medicines (VAMs) are medicines based on known molecules that address healthcare needs and deliver relevant improvement for patients, healthcare professionals, and/or payers.
New drug applications via the 505(b)(2) pathway in the United States and hybrid applications under Article 10(3) of Directive 2001/83/EC in the European Union are roughly equivalent regulatory pathways that allow existing drugs to be improved.
The US Leads the Market for Value Added Medicines
As we shared in a post earlier this year, the number of drug approvals in the US through the 505(b)(2) pathway has grown significantly in the past two years (40% in 2017 and 19% in 2018):
Products ranged from limited to high added value for patients or other healthcare stakeholders, and the vast majority of companies that obtained approval can be classified in two main groups:
- Specialty pharmaceutical companies (41%)
- Generic companies (41%)
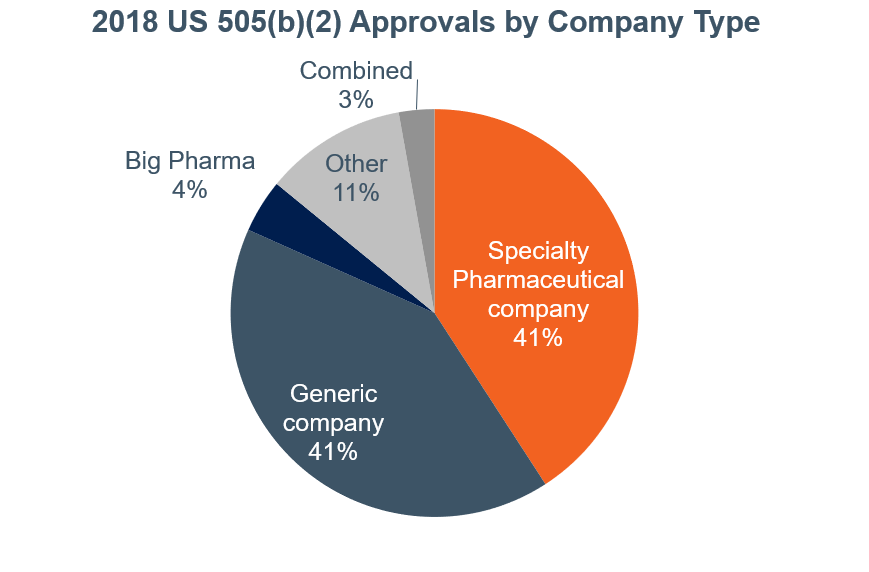
Generics companies have entered into the 505(b)(2) pathway for two primary reasons:
- To obtain an early market entrance to by-pass a specific patent. Examples of these types of products include new salts or new formulations based on new excipients.
- To obtain a product differentiation through added value for the patient or other health stakeholders. In this group we can find new formulations, new dosage forms, patient friendly dosages forms, combination products, etc.
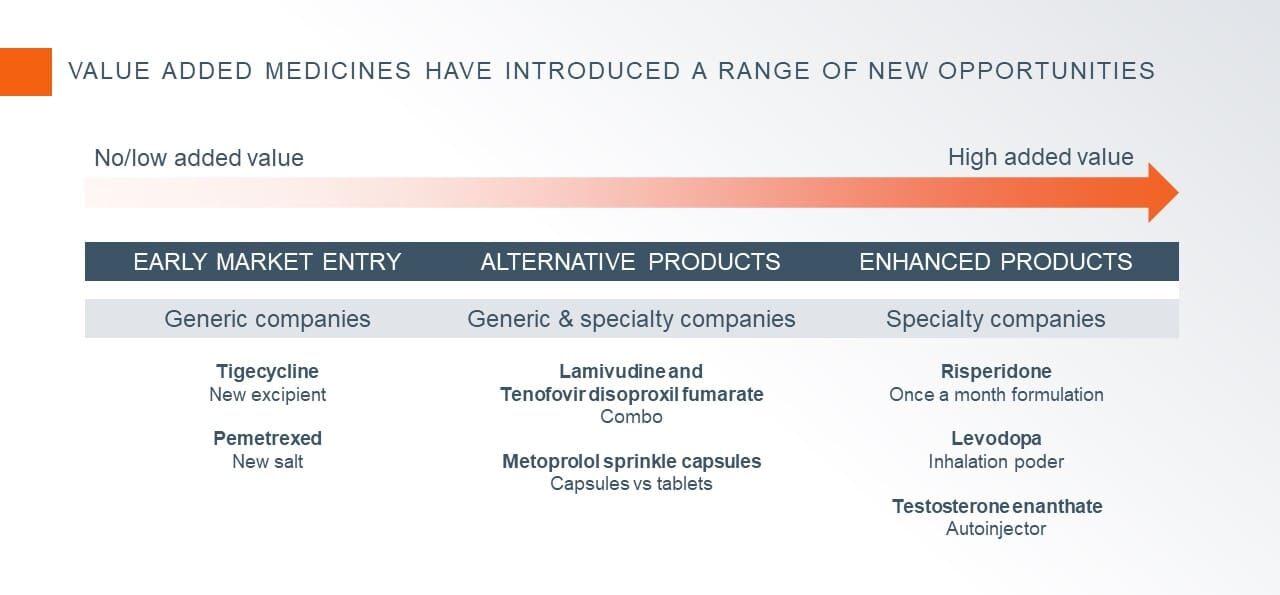
Key Success Factors for Value Added Medicine Development
The US market is evidence that generics companies are well-positioned to develop VAMs. To achieve success, there are several key factors to consider:
- Scientific – Does the science make sense? For instance, is the formulation stable and readily prepared? Is manufacturing scalable? Are active and inactive ingredients available and affordable?
- Medical – Does the product have a clear niche in the medical specialty? Is it effective for solving a unique problem or solving a problem in a unique way? Does it present an acceptable risk/benefit ratio? Is there evidence the product would be appealing to the proposed patient population?
- Commercial – Is there a viable market for the product? What is the potential for future competition or substitution? What is needed to ensure reimbursement? What is the optimal pricing?
- Regulatory – What clinical trials or other data will be required to gain approval? Can development be expedited? Would exclusive marketing rights (“exclusivity”) be available? What distinguishing information can be presented on the labeling for eventual promotional activity?
Understanding the Opportunity in Europe
In Europe, several key challenges have slowed adoption of VAMs as a strategic opportunity for pharmaceutical companies:
- Poor economics and lack of development incentives: Drug prices are generally fixed by the relevant health authorities, and pricing has not been sufficient to recoup R&D investments.
- Value added assessment: VAM might be alternatively perceived by health authorities as generics and a number of healthcare systems such as England, Poland, Italy and Germany do not have mechanisms to assess and recognize VAM benefits. In some countries, the level of proof required to demonstrate the benefit can be prohibitive (e.g., France, Sweden and Scotland).
- Regulatory pathway: Most VAMs are not approved through Hybrid applications under Article 10(3), requiring full regulatory submission dossiers and incurring high costs.
To address these challenges, a recently formed Value Added Medicines trade group, established as part of Medicines for Europe, is working to establish a sustainable market model that incentivizes R&D and access to these medicines in Europe.
In addition, some European healthcare systems are starting to introduce initiatives that drive a higher VAM reward. Belgium recently implemented a new system that protects VAMs from being compared to standard generics on price provided that the “value added” is justified by means of pragmatic scientific argumentation. In the UK, a tax exemption for VAM R&D costs has been proposed to lessen the investment required in generating the burden of proof (following in the footsteps of France where a tax remuneration for VAMs already exists), and an accelerated access pathway is being considered.
These initiatives are expected to build momentum and spur growth for the European VAM market. Premier Consulting will participate in the 3rd Annual Value Added Medicines Conference, on November 21 in Brussels. If you are interested in learning more about the Value Added Medicines market in Europe and shaping the path forward to fulfill unmet medical needs, we hope to see you in Belgium.