The Economics of Investigational Device Exemption (IDE) Studies: What You Need to Know About Medicare Coverage
Significant risk studies of medical devices require an investigational device exemption (IDE), which allows the device to be used in a clinical study to collect data on its safety and effectiveness.[1] Often, device developers wonder whether—and how—the cost of those investigational devices might be covered not only to offset the cost of development but also to enable access and accelerate innovation.
This article reviews the regulations governing charging or obtaining insurance coverage for an investigational device. We also discuss potential policy changes that may create an alternative, expedited pathway to payment and coverage for emerging devices and diagnostics.
Charging for Investigational Devices in IDE Studies
Under IDE regulations, sponsors are allowed to charge for an investigational device provided that the charge does not exceed an amount necessary to recover the costs of manufacture, research, development and handling of the device.[2] In their IDE application, the sponsor must state the amount to be charged, justify the proposed charges in the IDE application, and explain why the charge does not constitute commercialization.[3]
Understanding Medicare Coverage for IDE Studies
The Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (MMA) allows for Medicare coverage of the routine costs of care provided to Medicare beneficiaries in certain categories of Investigational Device Exemption (IDE) studies.[4] Effective January 1, 205, the Centers for Medicare and Medicaid Services (CMS) added criteria for coverage of IDE studies and changed to a centralized—rather than local Medicare Administrative Contractor (MAC)—review and approval of IDE studies. There are two types of coverage for IDE studies, if specific criteria are met:[5]
- Coverage A (Experimental), which allows coverage of routine care items and services provided in the study, but not of the device itself
- Coverage B (Nonexperimental/Investigational), which allows coverage of both routine care items and services and the device
The FDA is responsible for categorizing IDE-approved devices based on whether the available data demonstrates that initial questions of safety and effectiveness have been addressed.4 More information on the criteria used in the FDA’s categorization process can be found in FDA Categorization of Investigational Device Exemption (IDE) Devices to Assist the Centers for Medicare and Medicaid Services (CMS) with Coverage Decisions.
Of note, there are situations where it may be appropriate to change the Category from A to B if initial questions of safety and effectiveness have been resolved since the original categorization.
How to Request Medicare Coverage
The FDA communicates the assigned study category by sending the sponsors a formal letter. The sponsor then submits a request packet to CMS containing the following information:[3]
- A request letter describing the scope and nature of the IDE study. The letter should focus on how the IDE study meets each of the 10 regulatory Medicare coverage IDE study criteria (see Table 1).
- The complete FDA Category A or Category B approval letter
- IDE study protocol
- Institutional Review Board (IRB) approval letter
- National Clinical Trial (NCT) number
- Supporting materials, as appropriate
Table 1. Medicare coverage IDE study criteria3
| The principal purpose of the study is to test whether the device improves the health outcomes of appropriately selected patients. |
| The rationale for the study is supported by available scientific and medical information, or the study is intended to clarify or establish the health outcomes of interventions already in common clinical use. |
| Study results are not anticipated to duplicate existing knowledge |
| Study design is methodologically appropriate, and the anticipated number of subjects is adequate to answer the research questions(s) being asked |
| Sponsor is capable of successfully completing the study |
| The study complies with all applicable Federal regulations concerning the protection of human subjects |
| Where appropriate, the study is not designed to exclusively test toxicity or disease pathology in healthy individuals. Studies of devices measuring therapeutic outcomes may be exempt from this criterion only if the condition being studied is life-threatening and the patient has no other viable treatment options |
| The study is registered with ClinicalTrials.gov |
| The study protocol describes the method and timing of release of results on all pre-specified outcomes. |
| The study protocol describes how Medicare beneficiaries may be affected by the device and how study results are or are not generalizable to the Medicare beneficiary population. |
CMS has created a checklist to assist sponsors in submitting a complete package. CMS will review each complete submission within approximately 30 days of receipt. If the submission is not approved, the sponsor may submit revised protocols.
Expediting Coverage and Payment
According to a recent study, it takes an average of five years for medical technologies to achieve nationwide coding, coverage, and payment.[6] CMS is working with industry stakeholders to develop an alternate Medicare coverage pathway called Transitional Coverage for Emerging Technologies (TCET). The intent of this pathway is to:
- Facilitate early, predictable, and safe access to new technologies for Medicare beneficiaries
- Reduce developer uncertainty about coverage through early evaluation of the potential benefits and harms of their technology
- Encourage evidence development if evidence gaps exist
The proposed TCET pathway would use current national coverage determination (NCD) and coverage with evidence development (CED) processes to expedite coverage of certain Breakthrough Devices.
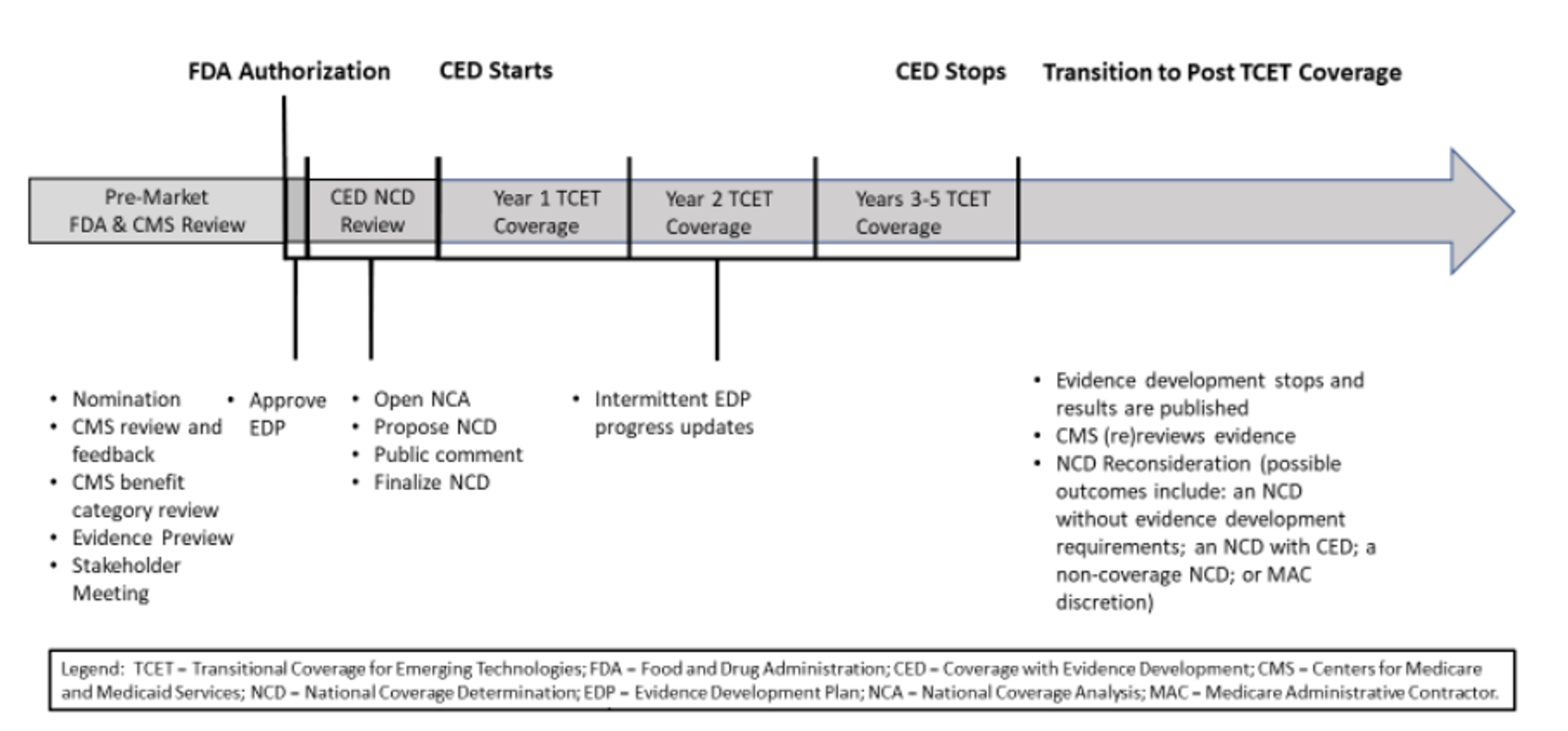 Figure 1. Proposed TCET pathway and timeline
Figure 1. Proposed TCET pathway and timeline
Key takeaway
Sponsors of significant risk device studies may be able to secure Medicare coverage for routine care items and services—or even their investigational devices—in their IDE studies. This coverage helps to increase access by removing a financial barrier that might otherwise discourage beneficiaries from participating in clinical studies. It may also help to enhance diversity, making study results more representative of the eventual end-user population. In addition, future policy changes may lead to the development of an alternate Medicare coverage pathway for certain Breakthrough Devices, which could expedite coding, coverage, and payment of innovative technologies.
To learn more about Medicare coverage of IDE studies, contact us.
Resources
[1] US Food and Drug Administration. Investigational Device Exemption. Available at https://www.fda.gov/medical-devices/premarket-submissions-selecting-and-preparing-correct-submission/investigational-device-exemption-ide.
[2] Code of Federal Regulations. § 812.7 Prohibition of promotion and other practices. Available at https://www.ecfr.gov/current/title-21/chapter-I/subchapter-H/part-812/subpart-A/section-812.7#p-812.7(b).
[3] Code of Federal Regulations. § 812.20 Application. Available at https://www.ecfr.gov/current/title-21/chapter-I/subchapter-H/part-812/subpart-B/section-812.20#p-812.20(b)(8).
[4] CMS. Medicare Coverage Related to Investigational Device Exemption (IDE) Studies. Available at https://www.cms.gov/medicare/coverage/investigational-device-exemption-ide-studies.
[5] US Food and Drug Administration. FAQs about Investigational Device Exemption. Available at https://www.fda.gov/medical-devices/investigational-device-exemption-ide/faqs-about-investigational-device-exemption.
[6] Stanford Byers Center for Biodesign Innovation Policy Program and Duke Margolis Center for Health Policy. The Need for Transitional Coverage for Emerging Technologies, March 28, 2022. Available at https://healthpolicy.duke.edu/sites/default/files/2022-04/TCET%20Webinar_Slides%203.28.22.pdf.