Seamless Clinical Trials: Why Didn’t We Think of That?
Seamless clinical trials have become the new buzz word in drug development since FDA Commissioner Scott Gottlieb promoted their use this month. But are they new, and which products are best suited to this style of clinical trial?
Oncology drugs are the obvious examples of products that are well suited to the adaptive design of seamless trials. However, for decades Premier Consulting has been designing innovative seamless trials with drugs for a broader range of diseases/conditions to save time and money. Fortunately for our clients, Premier Consulting already has the experience to design development programs that support Commissioner Gottlieb’s initiative.
Traditional Clinical Trials
We are all familiar with the traditional phases of full clinical development programs, in which each phase is designed to establish separate goals.
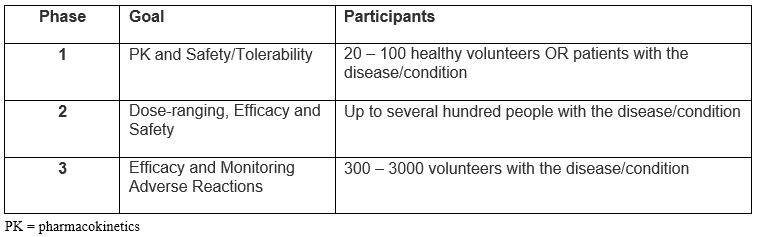
Source: Adapted from FDA Drug Development Process: Clinical Research
The traditional paradigm is modified slightly in oncology trials in which Phase 1 goals typically include safety/tolerability, and dose; Phase 2 explores drug activity in a variety of cancers; and Phase 3 studies compare the efficacy of drug with existing treatments1.
The Benefits of Seamless Trials
In contrast to traditional clinical trials, seamless clinical trials combine two or more phases into one adaptive design study. Decisions on how to ‘adapt’ the study are made after taking planned interim views of the data. For example, extra treatment arms may be included in earlier periods of the study to compare different doses, formulations, or disease states. The more successful treatment arms could then be extended with additional patient cohorts added, and less successful treatment arms could be discontinued at this point.
Products approved via the 505(b)(2) regulatory pathway can be particularly well suited to innovative adaptive trials designs. Seamless trials result in major savings in time and cost compared with traditional 3- or 4-phase programs. Additional benefits include longer-term safety data being accumulated earlier in the development program due to the continuity of patient exposure to drug from the earliest, and stronger data on dose selection, efficacy earlier in the program.
Are Seamless Trials New?
One of the best-known examples of a successful seamless trial includes the Merck first-in-human study of pembrolizumab (Keytruda®), a humanized monoclonal antibody specific for programmed cell death (PD-1) receptor-1 and its ligands. The study was initiated in 2011.
When the early results demonstrated impressive efficacy in patients with metastatic melanoma or non-small-cell lung cancer, additional cohorts were added to increase the sample size of these patients populations. In the end, this first-in-human trial enrolled more than 1200 people.
Within three years from study initiation, data from a cohort of patients in this study served as the basis for accelerated approval of Keytruda. Other data from the study has since been used to expand the indication to non-small-cell lung cancer, head and neck cancer, classical Hodgkin lymphoma, urothelial carcinoma, microsatellite instability-high cancer, and as of last Friday, 22 September 2017, gastric cancer.
However, Premier Consulting has been involved in the design of seamless trials all along (see examples below). This means we understand the challenges and benefits associated with these trials.
Can We Do Away with Traditional Clinical Trials?
Not so fast. Seamless trials work well for certain development programs and disease states, particularly oncology drugs. But they do raise issues, and may not be appropriate in all cases.
In an article from 2016 on seamless oncology development programs, the FDA notes the concerns from multiple stakeholders regarding the rapid proliferation of first-in-human trials enrolling hundreds or even thousands of patients1. Further, the FDA has concerns regarding appropriate statistical analysis plans, a defined end to the trial in terms of efficacy and futility, systems for rapid and effective communication with investigators, and regular updating of the informed consent during a trial.
The need for a mechanism for Sponsors to meet with regulators outside of the typical milestone by Phase meetings (e.g.. End-of-Phase 2) is apparent. As is the need for an independent data and safety monitoring committee to review data and make recommendations regarding changes to the trial’s conduct.
Which Development Programs Are Best Suited to Seamless Trials?
In the article authored by the FDA in 2016, it was proposed that seamless development programs drugs be reserved for products that are granted breakthrough therapy designation, and therefore the preliminary clinical evidence indicates a substantial improvement over available therapy. This is because Breakthrough designation allows for more frequent and more intensive interaction with the FDA.
In Commissioner Gottlieb’s recent address to the Regulatory Affairs Professionals Society Regulatory Convergence Conference, he mentioned that the success of seamless trials in oncology programs could be expanded to other products, such as immunological therapies and in drugs targeting specific molecular defects across a variety of disease states.
However, Premier Consulting has been successfully applying the seamless approach to a broader range of clinical studies to meet the needs of each Sponsor. Designing credible drug development programs that reduce drug development costs, timelines and program size has always been our goal. The former FDA reviewers on our team have a deep knowledge of how to design combined-phase studies that will meet regulatory requirements, to minimize patient enrollment, time, and costs.
Examples of Premier Consulting’s Seamless Development Programs
Premier Consulting evaluates each drug product’s development program for meeting regulatory requirements while minimizing unnecessary studies or steps. These goals are applied to all programs, and in many cases can be achieved with seamless trials.
Here is a quick sampling of the types of programs that Premier Consulting has been successful in designing and obtaining regulatory buy-in for:
- Oral extended release products: combining single-dose and multiple-dose pharmacokinetic studies
- Abuse-deterrent products: combining Category 2 pharmacokinetic requirements within the Category 3 human abuse liability study.
- Pivotal efficacy and safety studies: Using adaptive designs to combine Phase 2 and Phase 3 trials seamlessly with data review at intervals. These studies have been used to support registration (approval).
- Multiple indications: In one drug development program, two separate trials in closely related conditions that supported each other to obtain multiple indications in the product labeling.
- Injectable products: obtaining a clinical biowaiver to save on trial costs throughout the development program.
Premier Consulting relishes the chance to work on unique and challenging development programs. Our value is in finding innovative solutions to reduce the size and scope of development programs while meeting all regulatory requirements. Designing and overseeing seamless trials is just one tool that we use to deliver value. Contact us to learn more about new ideas for your development program.
Authors:
Angela Drew, PhD
Product Ideation Consultant
Ruth E. Stevens, PhD, MBA
Strategic Advisor
References
- Seamless Oncology — Drug Development. Prowell TM, Theoret MR, Pazdur R. N.Engl.J.Med. 2016 May 26;374(21):2001-3