Nonclinical Pulmonary Drug Delivery: Setting Up Your Inhalation Drug Study for Success
Most people know someone who uses an inhaler to prevent or relieve shortness of breath after exertion or during allergy season. Medicine has been delivered by inhalation for decades to treat human respiratory diseases such as asthma, chronic obstructive pulmonary disease, cystic fibrosis, and airway infection. Interest in the pulmonary route has grown in recent years because delivering drugs through the lungs allows direct systemic delivery and bypasses first-pass metabolism.
But it’s not that simple.
Pulmonary drug delivery is a complex challenge. Unlike other organs used in drug administration, the lungs are directly accessible via the airways. Pulmonary delivery is highly specialized, and despite our vast and growing understanding of respiratory diseases and aerosol technologies, developing new and improved inhaled medicines still presents a number of challenges, particularly in delivering the drug to its target.
The lung is an organ of exquisite structural complexity, with a tree-like airway structure composed of many specialized structures of different cell types. The primary functions of these structures are to get oxygen from the air we breathe into the body, remove carbon dioxide, and act as a first line of defense against airborne pathogens and particles.
The structure and function of diseased lungs, such as those in cystic fibrosis and COPD patients, are often altered dramatically. Lung disease can result in changes such as mucus hypersecretion or thickening, a narrowing or collapse of the airway, and fibrosis. All these changes make it more difficult for the drug to reach and be distributed throughout the lung tissue.
Delivering drugs to the lungs in nonclinical studies
Inhaled therapeutics are delivered to human lungs in two main forms: a dry powder or a liquid solution. Either form must use specialized devices to aerosolize the drug, allowing efficient inhalation and lung deposition. This also applies to delivery of drugs to animals when assessing the safety of novel inhaled drugs. Toxicity studies in animals require specialized exposure systems that use either rotating brush generators (for dry powder drugs) or nebulizers (for liquid solutions) along with a large amount of test compound for inhalation delivery to a large number of animals simultaneously. These systems allow the generation of aerosol particles with characteristics closely matching those produced by commercial devices used in the clinic. For maximum drug deposition, system parameters and conditions are set to maximize the number of respirable particles with an appropriate size distribution or mass median aerodynamic distribution.
However, in certain small studies, such as proof-of-concept pilot studies or those with a limited amount of drug, direct administration methods (e.g., nasal or intratracheal instillation or insufflation, oral or intratracheal aerosol delivery, oropharyngeal) are an option. These direct delivery methods always achieve a higher effective dose, but all suffer from non-homogeneous lung deposition and can never be directly compared to inhaled administered doses. Premier Consulting can help you select the most appropriate route of administration for your nonclinical program based on these considerations.
How much drug is delivered to the lung?
While the exact dose of drug products given to an animal using a capsule or syringe can be measured precisely, measuring the dose of an inhaled drug given to an animal is more technically challenging. The animal is exposed to an atmospheric concentration of the drug that it breathes in, effectively self-dosing based on its breathing frequency and tidal volume. This means that the delivered dose needs to be estimated from the total air volume inhaled for the duration of the exposure, the animal’s body weight, the aerosol concentration, and the size of the aerosol particle.
The following formula from Alexander et al. is commonly used to estimate the delivered dose during the exposure period:
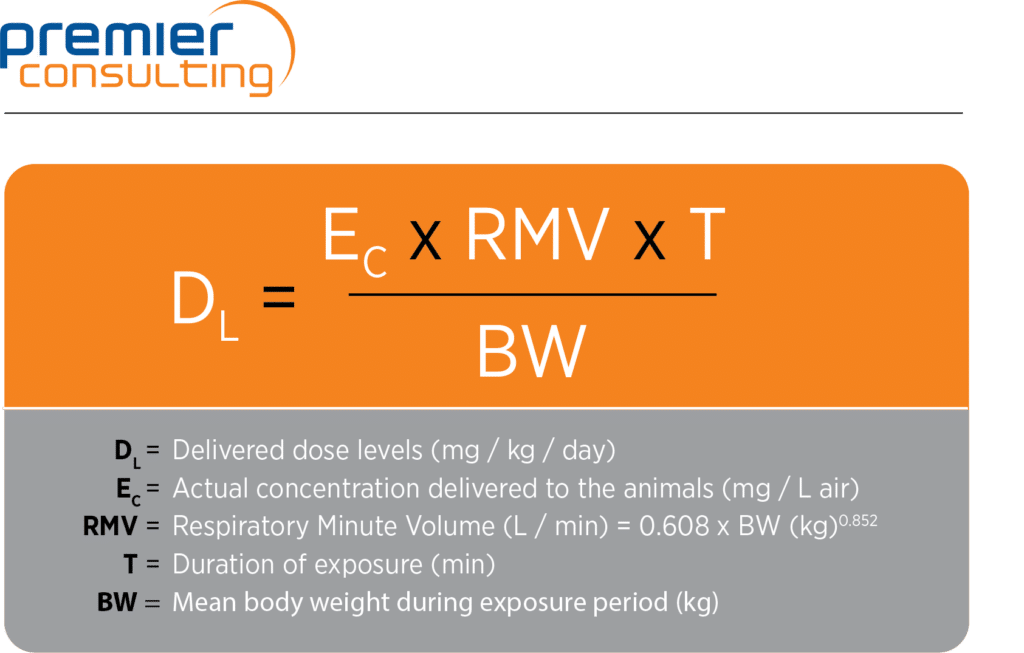
The delivered dose differs from the achieved dose (or the “deposited” or “respirable” dose) in the lungs. The achieved dose is calculated using the deposited or respirable fraction, which regulators generally assume to be 10 percent of the delivered dose in rodents and 25 percent in larger animals (dogs and non-human primates). These considerations should be factored in when planning the manufacture of the test compound required for an inhalation program.
Adverse effect vs. “normal” reaction to inhaled drugs
The “normal” lung response to inhaling materials can be characterized as a non-specific irritancy leading to some changes, including an inflammatory response and hypersecretion of mucus, and similar changes are commonly observed in nonclinical studies. However, not all toxicity of inhaled drugs manifests as morphologically observable injury. Functional changes may occur without associated changes in morphology, whether acute or subtle, such as those in ventilation and breathing patterns.
Therefore, discerning true adverse effects from normal physiological lung responses can be a major scientific challenge when interpreting toxicology findings. One of the primary difficulties in advancing these molecules into clinical trials is determining whether the observed lung findings in nonclinical studies represent safety concerns for humans. This is a critical step, as patients with diseased lungs may have increased susceptibility.
With deep nonclinical expertise, Premier Consulting provides solutions to help you design, execute, and manage your nonclinical program. In addition to our regulatory and strategy capabilities, Premier Partners delivers nonclinical study execution services from leading CROs, plus study management services at no additional cost. Thanks to our extensive experience with nonclinical inhalation programs, we are your ideal partner to ensure minimized risks, optimized programs, and flawless study oversight. Contact us today.
Author:
Nicolay Ferrari, PhD
Senior Director of Research
Premier Partners