Increasing Clinical Trial Success: Pharmacokinetic Modeling & Simulation to Optimize Dosing Regimens
In the complex and costly world of new drug development, pharmacokinetic (PK) modeling has emerged as a promising method for driving more efficient and cost-effective clinical trials. This scientific approach leverages mathematical models to describe the relationship between drug dose, concentration in bodily fluids, and time. By simulating these processes, sponsors can optimize dosing regimens and assess variability in drug response among individuals.
In this blog, we review the benefits of PK modeling and simulation in the drug development process, discuss how PK modeling enables safe and efficacious therapies, and present a case study demonstrating how this method supported key decision-making in setting a phase 2 study dosing regimen.
The key to accelerating drug development
PK modeling and simulation streamline drug development, improve the understanding of drug behavior, and enable the design of safer, more effective therapies. When applied correctly, this resourceful approach offers significant benefits throughout the product development lifecycle, leading to:
- Optimized dosing regimens: Simulation enables researchers to predict the drug’s behavior across various doses and dosing schedules. This facilitates the design of dosing regimens that maximize efficacy while minimizing adverse effects.
- Reduction in clinical trial costs and timelines: By predicting drug behavior in virtual populations, PK modeling reduces the need for multiple human trials, expediting the development process and saving resources.
- Improved safety profiles: Simulations can forecast drug concentrations at different tissue sites, helping to identify potential toxicities and refine dosing strategies to improve patient safety.
- Support for regulatory submissions: Regulatory agencies increasingly emphasize the importance of integrating modeling and simulations in drug development and its impact on decision-making and encourage the use of PK modeling and simulation as part of drug approval submissions. i
- Bridging studies and extrapolation: PK modeling allows for the extrapolation of data between different populations or dosage forms, reducing the need for additional clinical trials. For example, it can predict pediatric dosing based on adult data.
Safety and efficacy: Finding the right balance
In drug development, achieving a steady state is crucial for ensuring effective and safe drug therapy. The steady state is assessed by a combination of steady state, minimum and maximum plasma concentrations (i.e., Css, PKCmin, and Cmax) and area under the curve (AUC). It occurs when the rate of drug administration equals the rate of elimination. This results in a consistent drug concentration in the body’s plasma and tissues over time and is essential for maintaining drug levels within the therapeutic window, where the medication is effective without causing toxicity. Fluctuations outside this range can lead to subtherapeutic effects or adverse reactions, undermining treatment goals.
To accelerate the drug development process, PK modeling enables the prediction of plasma concentrations and AUC under repeat dose steady state conditions using data obtained from initial single dose studies and allows skipping preliminary trials that test multiple dosing regimens. If an unfavorable outcome is predicted from the modeling, the sponsor may elect to alter the dose, dosing regimen, or reformulate the product prior to conducting more robust phase 2 and/or phase 3 studies.
PK modeling in action: A case study
Premier’s expert consultants used phase 1 single ascending dose (SAD) PK data to model and simulate repeat dose steady-state conditions for once daily (QD, once every 24 hours) versus twice daily (BID; once every 12 hours) dosing regimens to determine if QD dosing, which is more favorable from a patient compliance perspective, is similar to BID dosing.
As shown in the figure, the simulations provided the PK profiles and key PK parameters for the two dosing regimens. Steady state was reached by Day 7 and the AUCs (1896 vs 1893 hr.mg/mL for BID vs QD) and average concentrations at steady state Css (79 vs 78 ng/mL for BID vs QD) for both regimens were similar. However, the Cmax as steady state for QD dosing was almost double of the BID regimen and Cmin demonstrated a greater drop for QD.
This analysis importantly demonstrated that the time to steady state, AUC, and Css was similar for both regimens. For some drugs, particularly ones with a narrow therapeutic index, a greater Cmax demonstrated in the QD regimen can present a safety issue; however, in this particular case, the higher Cmax was not a safety concern, and the sponsor proceeded with the QD regimen to increase patient acceptance and compliance.
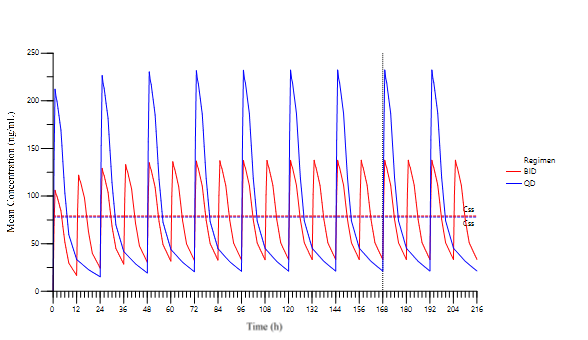
In the absence of actual steady state data, the modeling/simulation results allowed for timely, confident decisions to be made about the dosing regimen justification for the sponsor’s planned phase 2 study and overall clinical development plans.
Driving smarter drug development
PK modeling is an indispensable tool in drug development, enabling data-driven decisions that improve the likelihood of success. Its ability to predict drug behavior, optimize dosing, assess safety, and support regulatory submissions makes it a fundamental component of modern clinical development.
For support on leveraging PK modeling and simulation in your drug development plans, contact us.
[1] Miller R, Ewy W, Corrigan BW, Ouellet D, Hermann D, Kowalski KG, Lockwood P, Koup JR, Donevan S, El-Kattan A, Li CS, Werth JL, Feltner DE, Lalonde RL. How modeling and simulation have enhanced decision making in new drug development. J Pharmacokinet Pharmacodyn. 2005 Apr;32(2):185-97. doi: 10.1007/s10928-005-0074-7. Epub 2005 Nov 7. PMID: 16283534.