Importing Investigational Pharmaceuticals to the U.S.? Changes Are Coming
The United States government is making ongoing, concerted efforts to improve the importation process for many products, including pharmaceuticals and medical devices. It’s important to stay on top of the new importation changes and their effect on business. Foreign biotech, device manufacturers, API producers, and pharmaceutical companies should pay attention as the changes go into effect in December 2016. These changes will affect companies that manufacture drugs or devices in India or China, for example, for use in clinical trials in the US. Unnecessary delays in clinical studies can be avoided by becoming familiar with the new importation changes now.
Government agencies are phasing out the Automated Commercial System (ACS) for the newer Automated Commercial Environment/International Trade Data System (ACE/ITDS). The mandatory transition date to ACE is December 2016. This project is mandated by the Security and Accountability for Every Port Act of 2006 and the Executive Order on Streamlining the Export/Import Process for America’s Businesses, issued February 2014.
What is ACE?
ACE/ITDS is a single access point whereby industry can electronically submit all data required by various government agencies involved in international trade.
Before ACE, importers had to submit the same information to multiple government agencies, including US Customs and Border Protection (CBP) and FDA, multiple times, through processes that were sometimes paper-based and manual. The ‘single-window’ concept is aimed to streamline the process for importers and government agencies.
One of the missions of US Customs and Border Protection (CBP) is to facilitate lawful international trade. As part of this responsibility, CBP performs an administrative role in the importation of FDA-regulated commodities.
A pilot program to facilitate the ACE transition began in August 2015 for drugs, medical devices, and biologic products arriving at the ports of Philadelphia, Baltimore, and Otay Mesa. After the success of the first phase, the pilot program was expanded to all commodities, and for all ports. The purpose of the pilot was to test electronic filings made through ACE, and to assist users in transitioning to ACE ahead of the mandatory transition date of December 2016.
Process for Filing and Processing of an Import Application for an FDA-regulated Product
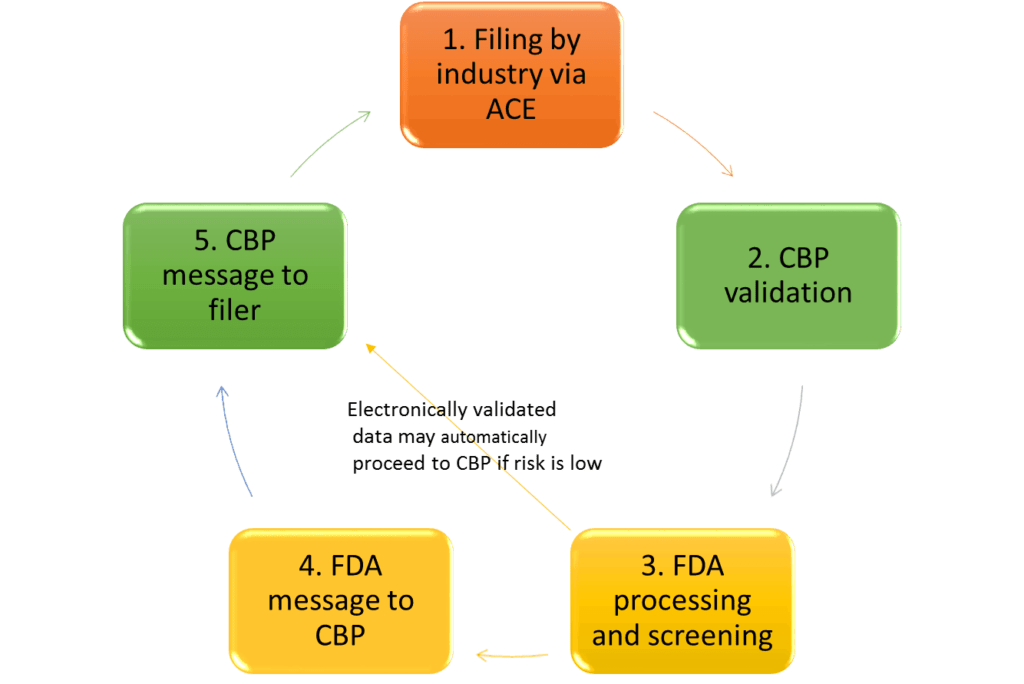
ACE = Automated Commercial Environment; CBP = Customs and Border Protection
Step 1: performed by customs brokers (licensed by CBP), self-filing importers, or carriers.
Steps 2 and 3: result in an error message back to the filer if data is missing or incorrect.
Step 4: may be skipped for electronically validated data depending on the risk ranking of the submission.
Source: Adapted from ACE: Specific Information for Pharmaceutical Importations, John E. Verbeten, US FDA Division of Import Operations, Central Atlantic States Association of Food and Drug Officials Pharmaceutical Industry Seminar April 25, 2016.
As a result of an FDA entry review, a decision is made to release the product, request more information/perform an examination, or detain the product based on the submitted information or import alerts. If information in the ACE submission does not match FDA sources, the product will likely be detained as misbranded, as per Federal Food Drug and Cosmetic Act Section 302(o). Detentions occur when the declared manufacturer, product combination, or importer is not listed or not part of an approval, and no exemption exists.
This process applies to investigational and approved products. In both cases, permission to import is product specific and must include dosage form, strength, and foreign source. For an investigational drug, the IND must be in effect (open) and the importer or consignee must be the IND Sponsor, their agent, or the investigator/s. Any exemptions for approved drugs must be claimed by the filer at the time of entry into ACE.
All foreign drug importers must also register with the FDA and provide the contact details for a US agent that can facilitate communications with the FDA, as per 21CFR §207.40. The US agent must reside or maintain a place of business in the US. The importer/agent must provide FDA with product registration and listing information including labels and labeling in English.
What will change with ACE?
Entry information for BCP, FDA, and other partner government agencies will be entered once into one system. Messages from each agency will be sent back to the filer. This should reduce entry and processing time.
Complete data will be required by FDA (and other government agencies) at the time of submission.
Affirmations of Compliance (AoC) will be required. AoC information includes Drug Registration (REG), Drug Listing (DLS), Drug Application Number (DA; ie. NDA or ANDA number), or Investigational New Drug Number (IND). Currently, AoCs are not required as entry reviewers manually verify this information anyway. However, including AoC information in ACE will allow an automatic ‘proceed’ assessment in many cases that will speed review times. Automatic prompting for this information in ACE should help prevent the most common entry error found when transmitting data to the FDA: submission of incorrect AoC information.
Importers and brokers need input only name, address, and Dun & Bradstreet (D&B) Data Universal Number System (DUNS) number instead of detailed company information. DUNS numbers can be requested or checked via the FDA ITDS/DUNS Portal. Using DUNS numbers for Manufacturer, Shipper, Importer, and Delivered To Party will save entry time and help avoid the second most common entry error: submission of incorrect manufacturer information. Note that the manufacturer refers to the manufacturing facility not the corporate office.
Of course you can also avoid error messages and delays to the FDA entry decisions by providing your entry filer with correct FDA Product Codes and Intended Use Codes, and Accurate Product Description (API and proprietary name) information.
What Should Importers Be Doing Now?
- Read the quick guides on the DUNS portal, specifications and requirements for filing through ACE, and contacts for additional support on FDA’s ACE website
- Talk to your entry filer about filing through ACE.
- If you are a filer:
- contact your CBP client representative to test in CBP’s certification environment prior to participating in the ACE pilot
- View the guidances, support videos and ACE webinars on CBPs website
- Participate in the ACE pilot
- Be ready for the mandatory transition to ACE in December 2016.
To learn more about importation of pharmaceuticals or medical devices, or how Premier Consulting can help oversee and facilitate the successful and timely completion of your clinical and nonclinical studies, contact us.
Author:
Angela Drew, PhD
Product Ideation Consultant