Applying ICH Q14 in Pharmaceuticals Part 2: The Benefits of an Enhanced Approach in the Lifecycle Management and Post-Approval Changes of Analytical Procedures
In 2022, ICH Q14 officially introduced the enhanced approach1 in analytical development as an alternative path to the minimal approach, the benefits of which are worth exploring further. Analytical data is essential in demonstrating the safety and quality of pharmaceutical products and therefore the data should be reliable. A prerequisite for data reliability is a robust and fit-for-purpose analytical method that performs consistently within predefined criteria and per the established performance characteristics throughout the product’s lifecycle. Prior knowledge, development data, and risk assessments, along with analytical method robustness, should all be components of an overall control strategy, which must be established prior to method validation and confirmed based on validation results and conclusions. Until recently, this approach, otherwise known as enhanced1, was the least preferred for most small and medium size companies due to concerns associated with cost efficiencies. However, this approach does not have to be expensive to be successful, it just needs to be purposeful.
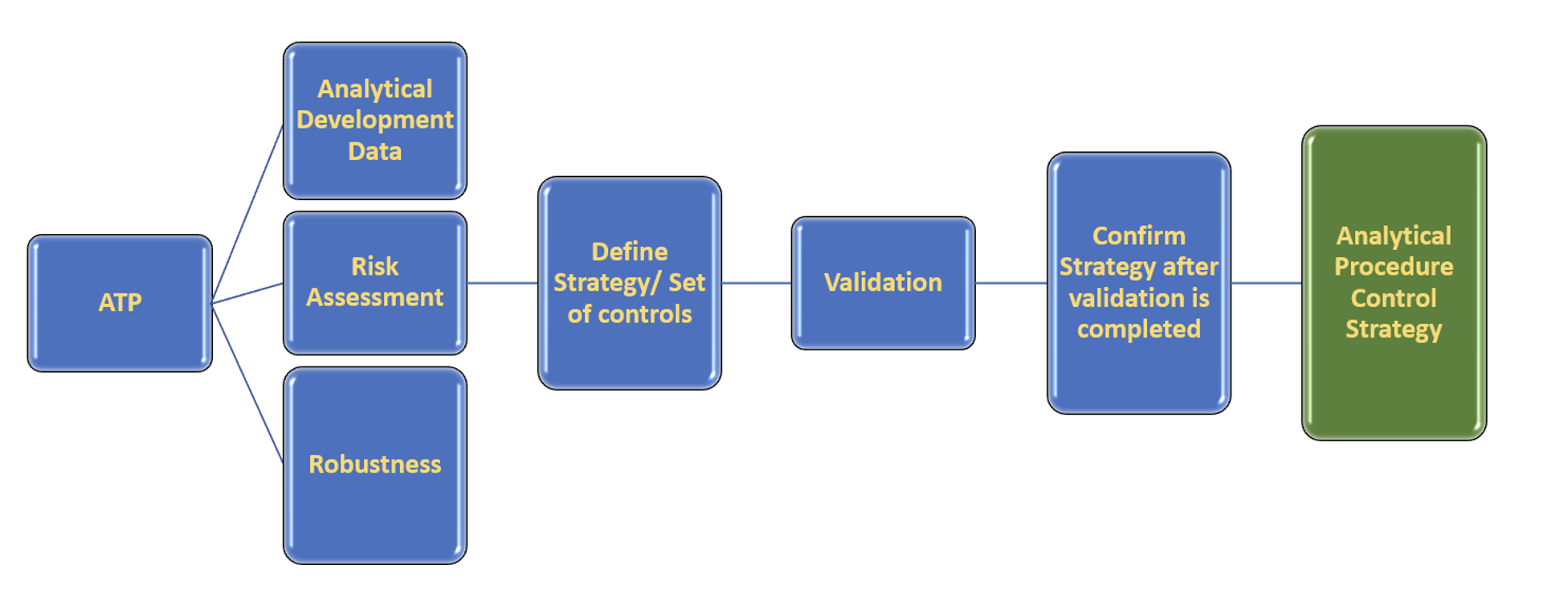 Figure 1: Elements leading to Analytical Procedure Control Strategy.
Figure 1: Elements leading to Analytical Procedure Control Strategy.
Approaches to Analytical Procedure Development Considering Lifecycle Management
When developing an analytical procedure, two main approaches are recommended per ICH Q141; the minimal and the enhanced approach. The minimal approach has been the default choice for years by many Sponsors and, as the name implies, it includes a minimum amount of information that is acceptable to the Regulatory Authorities. However, it creates a rigid regulatory space which restricts analytical method updates during development and post-approval.
The enhanced approach on the other hand provides a systematic way of generating knowledge as an analytical procedure evolves throughout its lifecycle. It is doing so by gathering and understanding information on the process and its parameters, by identifying analytical procedure factors and operational steps with potential impact on its performance, and by identifying and prioritizing analytical parameters to be investigated experimentally. For this plan to be set in motion and to be successful, several tasks must be performed during development in addition to the minimal approach. These tasks may include evaluation of prior knowledge, conducting risk assessments, defining an analytical target profile (ATP) and an analytical procedure control strategy, and conducting univariate or multivariate experiments. Additionally, they may include defining a lifecycle change management plan with established conditions (ECs), proven acceptable ranges (PARs) or, if so decided, method operational design regions (MODRs). It is evident that even within the enhanced approach there is space for flexibility depending on whether a concise or an extensive application of the enhanced approach is desired.
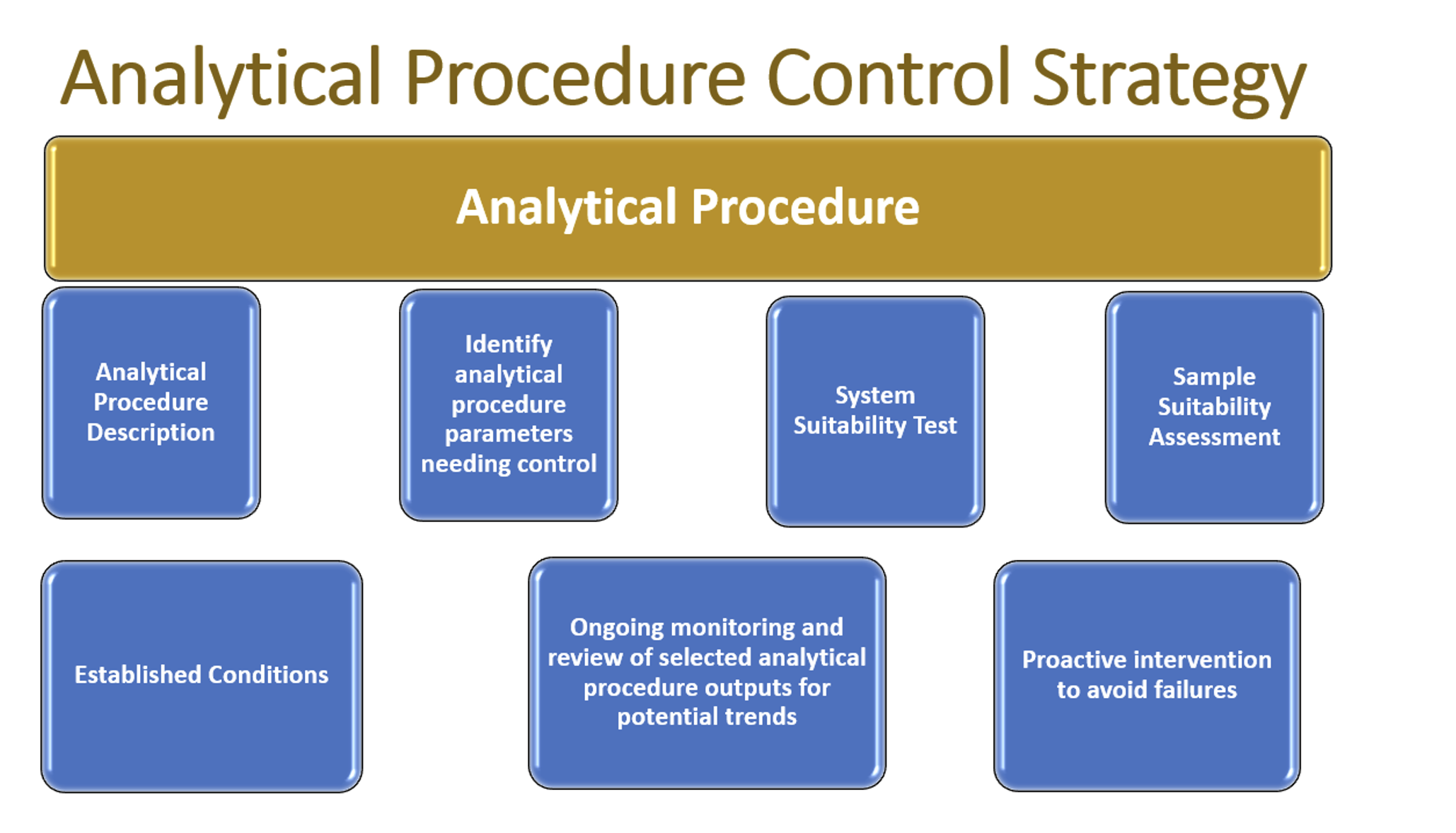 Figure 2: Components of the Analytical Procedure Control Strategy.
Figure 2: Components of the Analytical Procedure Control Strategy.
Development of multivariate analytical procedures can potentially be part of the enhanced approach. While univariate experiments of a single parameter can help identify PARs for the analytical procedure, multi-variate experiments examine the ranges for the relevant parameters and their interactions if data is generated by use of samples with appropriate variability. The extent of experiments, however, must be planned efficiently, to make the most of them in terms of cost and gained insight. It is not always practical, necessary, or possible to validate the entirety of a MODR. Typically, the outcome of a risk assessment feeds into the design and extent of the studies required to support a change. Establishing and receiving approval on MODRs, however, allows a Sponsor to make changes within the established parameter range without prior approval from or notification to regulatory authorities.
Why Change?
Changes to analytical method procedures throughout a drug substance’s or a drug product’s lifecycle are common. The financial and regulatory impact of such changes post-approval, however, depends on various factors, such as:
- the level of understanding of the analytical procedure: is it comprehensive?
- the risk management plan that has been put in place: is it exhaustive and continuous?
- the analytical method adherence to predefined criteria for performance characteristics: does it adhere to criteria for performance characteristics as described in the ATP?
Being able to answer these questions is one of the benefits of implementing the enhanced approach during analytical method development. Sponsors tend to be concerned that the enhanced approach may lead to money depletion and unnecessary regulatory scrutiny. Contrary to this belief, the enhanced approach can reduce costs and achieve regulatory efficiencies, if planned and conducted in a deliberate and systematic manner. A well-designed systematic approach (Figure 1) combined with well-defined and meaningful study designs using prior knowledge, may allow continual optimization of the analytical method as development progresses, without necessarily requiring full revalidation or prior approval.
Some Sponsors might question the need for method optimization and prefer a more rigid approach, staying within strict parameters and performance criteria throughout the method’s lifecycle. In an analytical lab environment, however, there are multiple reasons why this may not be feasible. First, unforeseen events causing deviations are possible even at facilities with strict compliance to GMP and GLP principles. Understanding the impact of the deviation often requires a robust analytical method control strategy to address the critical question if the analytical test results are reliable. If the test results are not deemed reliable, this may lead to data and drug product rejection, waste of material, time and resources, and potential delays on drug product development and market availability. Additionally, as historical data collection increases, and, consequently, product and process knowledge, the likelihood of having to deal with a need for method optimization also increases. The benefits of implementing an enhanced approach during analytical method development can be capitalized not only during product development, but also in post-approval changes, because they offer reliable tools that help define the impact of the change. Where a change is deemed major or moderate through the lenses of a minimal approach, the enhanced approach can provide an opportunity for more efficient regulatory approaches to post-approval changes because the reporting category can be downgraded based on justified lower risk.
Benefits and Efficiencies
The enhanced approach encourages the use of prior knowledge to eliminate redundancies. Prior knowledge may originate from internal libraries and knowledge repositories such as proprietary information or analytical data and experience, but it can also be external knowledge from reliable resources, such as peer-reviewed scientific publications or commonly acknowledged scientific principles. During routine use and monitoring of a procedure, additional data becomes available which allow to assess the performance of the method and compliance with the ATP. A well-established analytical procedure has the potential to be applied to multiple products with minimal or no modification of measurement conditions.
On certain occasions, the validation program may be reduced or streamlined based on data generated on analytical procedure performance characteristics from development studies. Statistical data or robustness data from well-designed development studies may be referenced, thereby eliminating the need to repeat these studies during validation. From a similar perspective, development studies and validation programs for a new application of well-established analytical procedures, may be reduced or streamlined based on previously generated data and knowledge as supported by established scientific principles and comprehensive risk assessments.
Once the relationship between analytical procedure parameters and performance is understood better, it is easier to decipher which factors require controls and which procedure parameters can tolerate wider ranges. Consequently, a well-defined, limited set of ECs may be identified, as opposed to having to assign an extensive number of ECs with rigid parameter ranges and set points when following the minimal approach.
CMC Lifecycle Management and Post-Approval Changes of Analytical Procedures
Understanding how to present information on analytical procedure development and analytical methods pre- and post-approval is essential. Incorporating information within the electronic Common Technical Document (eCTD) during development will help establish a historical sequence of events, explain and justify updates, showcase acquired knowledge, and support post-approval changes. The following information must be provided in regulatory CMC submissions in a phase appropriate manner:
- Description of the analytical procedures for drug substance and drug product, supportive information, and identified ECs.
- Performance characteristics and acceptance criteria as described in the ATP, MODRs, and PARs for analytical procedures.
- Verification or validation of the analytical procedures as applicable and supportive information to justify the analytical procedure control strategy.
- Development data for complex analytical methods, such as dissolution.
- Changes to analytical procedures throughout the product lifecycle (e.g. modification of existing procedures or replacement of procedures).
- Description and verification or validation of other analytical procedures used as part of the control strategy, e.g. for excipients or in-process tests.
During a product’s lifecycle, analytical procedure knowledge and continual improvement most often lead to either a necessary or a beneficial change based on performance evaluation and data trend analysis. The change can be minor or major, but the motive behind any change is typically the same, i.e. improvement. Aside from deep analytical procedure understanding and identification of ECs, which stem from the enhanced approach and set the basis for a reliable justification of the risk assigned to the intended change, there are some additional tools that enable this change. Specifically, Post-Approval Change Management Protocols (explaining how future changes will be managed), Product Lifecycle Change Management documents (enabling regulatory communication about potential post-approval changes) and Pharmaceutical Quality System documentation (of all changes including those not requiring regulatory submission) can be essential in managing potential changes while remaining regulatory compliant. Proper use of the enhanced approach and the existing tools for product lifecycle management should enable a more effortless evolution of the analytical procedure throughout its lifecycle.
Conclusion
Choosing the preferred approach of analytical procedure development as early as possible is very important for Sponsors to make the most of the data and information collected during development and to set a regulatory roadmap for the future. Before choosing between a traditional (minimal) versus an enhanced approach it is beneficial to understand efficiencies and costs (direct, indirect, immediate, and prospective) associated with either choice. It is also important to understand that the more Sponsors and regulatory bodies learn from applying the enhanced approach, additional guidance, training materials and workshops are expected to help gain a deeper insight into this type of approach for the purpose of providing more regulatory flexibility when changes are necessary.
Across every phase of product development, CMC experience is essential in achieving a product that meets regulatory standards and avoiding unnecessary development costs and timeline delays. Premier Consulting has deep technical and regulatory CMC expertise and has successfully supported multiple projects at all stages of development. Contact us today to find out how we can support your program.
Author: Marianthi Karakatsani, PhD, RAC, Director, Regulatory Affairs, CMC
Reviewed by: Eric Leblanc, RAC, Associate Director, CMC Services
References:
1 International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. ICH Harmonised Guideline. Analytical Procedure Development Q14. Available at https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q14-analytical-procedure-development