Juvenile Nonclinical Studies: What’s Involved, and How do I Proceed?
You’ve determined that you need to perform juvenile animal toxicity studies as part of your nonclinical program. The next step is understanding why these studies are required and how they should be designed.
Why are juvenile nonclinical studies needed?
Drug development programs use safety data generated from adult clinical trials, which are supported by nonclinical studies in adult animals. These studies assume that pediatric patients (neonates to adolescents) will show drug toxicity profiles similar to adults and respond to therapy in the same way. However, there are physiological differences between adults and children that may predispose pediatric patients to toxicities not observed in adults, and some of those differences are substantial:
- Significant ongoing organ and tissue postnatal growth and development in the neurological, cardiopulmonary, and immunologic systems
- Differences in drug pharmacokinetics, absorption, distribution, metabolism, and excretion due in part to differences in total body water volume and lipid partitions to changes in drug metabolizing enzymes
- Differences in pharmacodynamics (i.e., drug effects and mechanism of actions), which can affect the drug’s efficacy potential
When are juvenile nonclinical studies needed?
According to the FDA guidance Nonclinical Safety Evaluation of Pediatric Drug Products, sponsors must perform juvenile animal toxicity studies if the existing nonclinical toxicity data do not support the proposed clinical trials in pediatric populations. Depending on age, the organ systems in pediatric patients most at risk for drug toxicities include the brain, kidneys, and lungs, along with the reproductive, immune, skeletal, hepatobiliary, and gastrointestinal systems. However, most juvenile animal studies requested by regulatory agencies are for drugs being developed for indications involving the gastrointestinal, cardiovascular, endocrine, and central nervous systems.
Typical nonclinical reproductive toxicity studies focus mainly on drug-related effects during prenatal development, with limited postnatal evaluations. Juvenile toxicity studies focus on drug-related effects during postnatal exposure. Juvenile toxicity studies span the gap between the in utero perinatal/postnatal animal reproductive studies and the adult animal multidose toxicity studies.
How are juvenile animal toxicity studies designed?
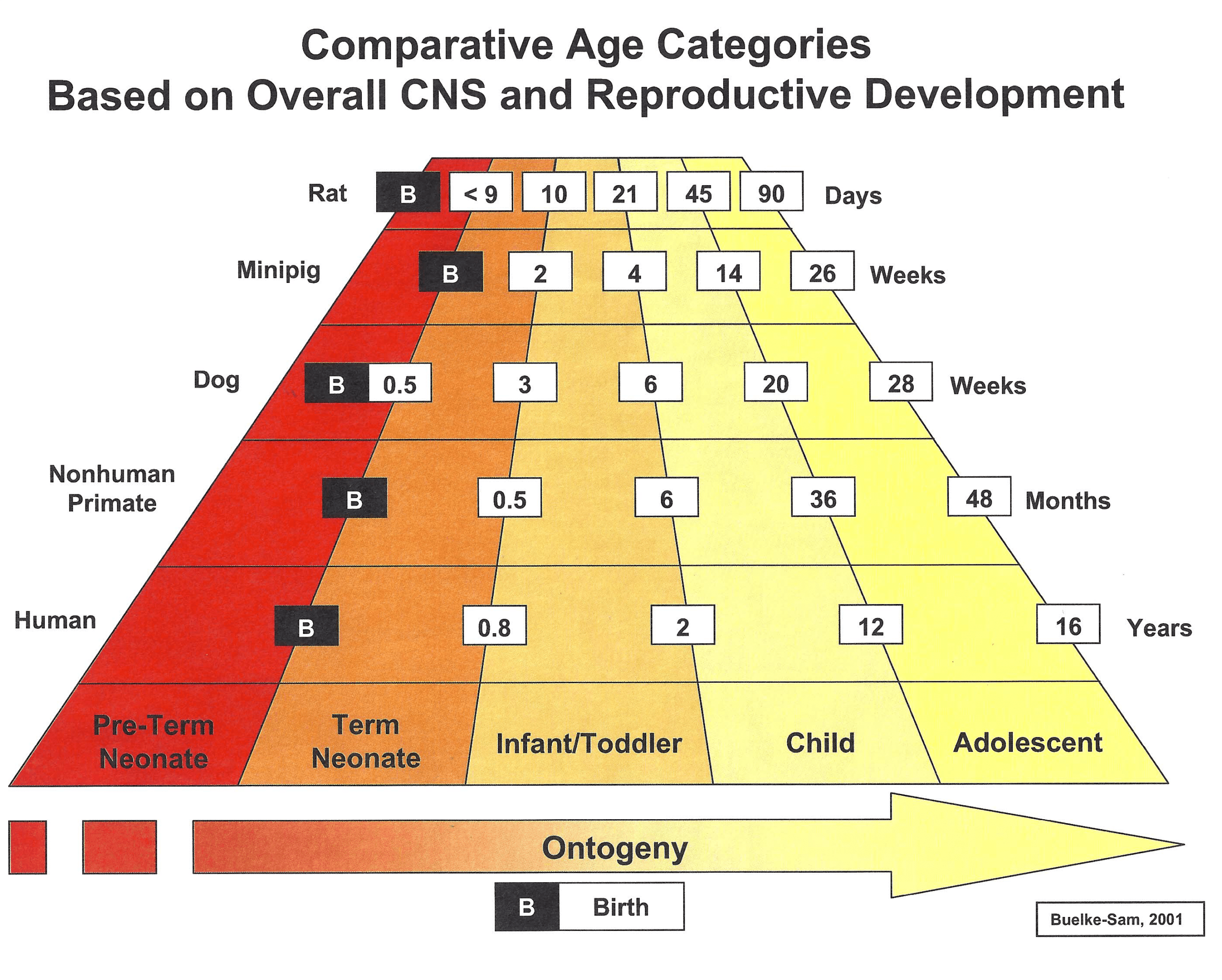
Sponsors must be able to justify their juvenile toxicity study designs — especially the animal species and age at the start of dosing — with respect to the disease indication, the route of administration, and the age of the patient. Additionally, sponsors should always clear study protocols with regulatory agencies before beginning the studies. The figure below illustrates the correlation between the age of various animal species and that of human pediatric patients based on the overall central nervous system and reproductive phases of growth and development. Age/species correlation to other human organ systems will vary.
Sponsors should consider a number of factors when designing a juvenile animal toxicity study:
Animal species, age, sex, and number: These are generally the most important factors for a scientifically sound study that supports a clinical pediatric study. Based on the site of pharmacodynamic actions of a drug, the age and sex of the animal species needs to correlate with the age and sex of the patients being treated. Typically, the rat is the species of choice, but other species are sometimes preferable based on species-specific receptor binding, sensitivity, metabolic similarity to pediatrics, limitations in blood volumes, and pharmacodynamic characteristics. However, there can also be limitations with other models, such as availability of the species, cost, and required study length.
Route of administration, dose levels, dose schedule, and dose formulation excipients and characterizations: Drugs typically should be administered to animals via the same route and on the same schedule as will be followed in the pediatric clinical trial, though sometimes a different route of administration is required. For example, while a drug can be administered clinically to neonates via intravenous infusion, it is impossible to do so in neonatal rat pups. In this case, subcutaneous or intraperitoneal administration may be an alternative route of administration. A nonrodent species that can be dosed intravenously also could be selected.
Toxicologic endpoints and biomarkers: Sponsors need to assess what in-life or post-mortem parameters to evaluate in relation to the human-to-animal comparisons of developmental periods. Since the juvenile studies will be performed under good laboratory practice (GLP), biomarker assays need to be validated under GLP as well.
Biological specimen volumes and collection methods: Depending on age and species, animal models often present limitations on sample volumes and when the samples can be collected
Ancillary toxicity studies: Additional tests may need to be incorporated into your juvenile study for evaluation of organ system functionality. For example, functional observation battery tests, motor activity and auditory startle tests, and learning and memory tests may be warranted for drugs that affect the neurological system.
Post-treatment periods: Delayed or non-reversible toxicities will need to be assessed.
Effects of immature animal use: The impact of dosing and handling immature animals should be considered.
Modification of perinatal/postnatal reproductive study design: It is possible that a juvenile toxicity study could be incorporated into your perinatal/postnatal reproductive animal study.
With deep nonclinical expertise, Premier Consulting provides solutions to help you design, execute, and manage your nonclinical program. In addition to our regulatory and strategy capabilities, our Premier Partners solution delivers nonclinical study execution services from leading CROs plus study management services at no additional cost. Contact us to learn more.