Tropical or Rare Pediatric Disease Priority Review Vouchers: Update and Use of the 505(b)(2) Pathway
While the United States market for drugs and biologics targeting tropical or rare pediatric diseases is very small, developing novel therapies for patients in these underrepresented groups can be advantageous for both patients and Sponsors. A number of incentives exist to encourage companies to apply for approval for these types of products, including orphan drug designation and priority review.
One valuable incentive provided by the FDA is the possibility of obtaining a priority review voucher (PRV), for Sponsors to consider in the development of drugs in the areas of drugs for tropical or rare pediatric diseases. A priority review voucher can be used on a subsequent application to speed the review process, and is an asset that can be sold or transferred an unlimited number of times to other companies.
Sponsors with a priority review voucher may use it for drug products targeting these diseases not only under the traditional 505(b)(1) pathway, but also under the 505(b)(2) pathway. The 505(b)(2) approval pathway allows for some information required for NDA approval to be obtained from studies not conducted by or for the applicant. If the 505(b)(2) pathway is used toward NDA approval, it can further reduce the development costs, thereby increasing the overall profitability of the program.
Priority Review Voucher Criteria
In our previous blog on the PRV program for tropical and rare pediatric diseases “Priority Review Vouchers: A Big Carrot for Hungry Companies,” we outlined the background of the program and rules for classifying a drug to treat tropical or rare pediatric diseases. Briefly, the PRV program was established to spur development of new drug and biological products for tropical diseases which, although rare in the United States, pose a direct threat to the health of Americans through the increasing use of intercontinental jet transport, immigration, tourism, and military operations. In 2012, the PRV program was also implemented to incentivize the development of new therapies for rare pediatric diseases. For a drug to qualify for the PRV program it must:
- be a new molecular or chemical entity,
- treat one of 19 tropical diseases or a rare pediatric disease,
- and qualify for priority review.
The qualifying application is issued a PRV at the time of marketing approval. While the tropical disease PRV program does not have an expiration date, the rare pediatric disease PRV program is set to expire on October 1, 2016 (after being extended once by Congress).
Acquisition and Fate of Priority Review Vouchers to Date
At the time of our previous blog in March of 2015, six PRVs had been granted (3 tropical, 3 rare pediatric). In the last year, perhaps in response to the scheduled expiration of the rare pediatric disease PRV program, another 3 rare pediatric disease PRVs have been granted (see Table 1). Of these three, one was approved under the 505(b)(2) pathway, and the other two were biologics. Taken together, of the 9 total PRVs granted, 2 have been granted under the 505(b)(2) pathway (both for rare pediatric diseases), 3 were granted under the 505(b)(1) pathway, and 4 were granted through biologics license applications (BLA).
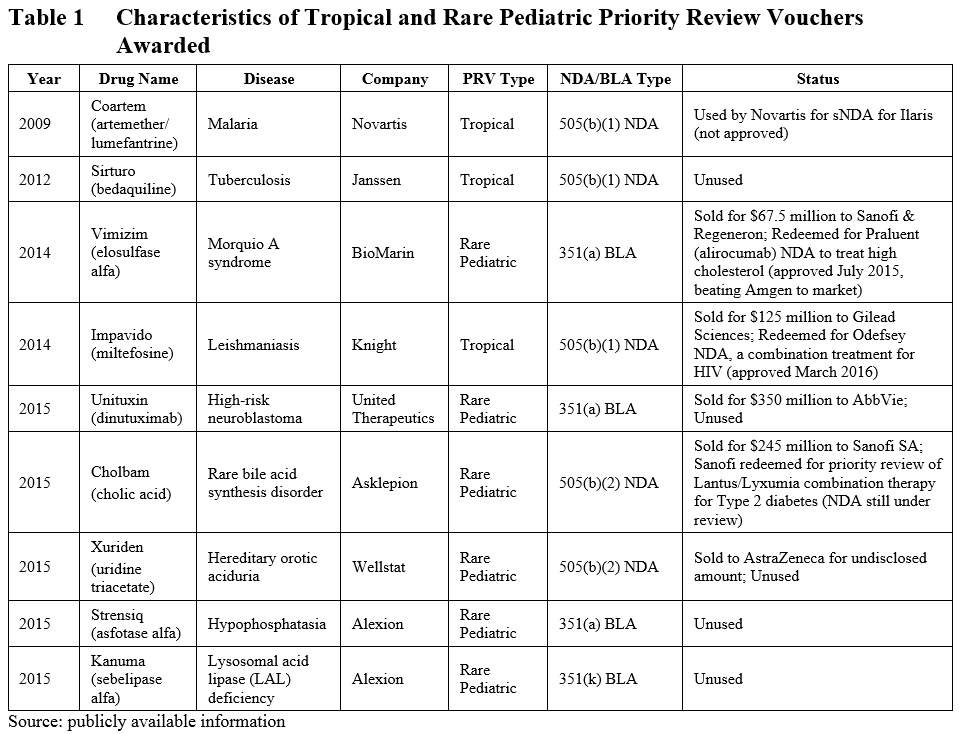
The advantage of a PRV is that it can be applied toward any application and will reduce the FDA review time by 4 months. This has made the PRV a highly sought-after commodity in the pharmaceutical field, as evidenced by the high price of PRV sales (as much as $350 million).
A search of the publicly available information on PRVs reveals that of the five PRV’s that have been sold, one was redeemed by Sanofi and Regeneron to expedite the review process of Praluent to treat high cholesterol (ahead of competitor Amgen), and another was used by Gilead to bring Odefsey (a combination product to treat HIV) to market faster.
Currently, another PRV has been redeemed by Sanofi to expedite the review of an NDA for a combination therapy for type 2 diabetes in an apparent attempt to beat Novo Nordisk to market, though that application is still under review. Of the remaining PRVs granted, one was redeemed unsuccessfully by Novartis to support a supplemental NDA application, and five have yet to be redeemed.
PRV Program Successes
While the future is uncertain for the rare pediatric disease PRV program (which is set to expire in the October 2016), the tropical disease PRV program will continue. Overall, this program, which provides incentives for the development of needed therapies for rare diseases has been successful. In particular, the benefits of rare pediatric drugs developed through this program (which are outlined in their respective summary basis of approval documents) include: improved quality of life and overall survival of patients compared to the previous standard of care.
Specifically, Vimizim has provided improved endurance for pediatric patients with Morquio A syndrome, for which only symptom-based treatments were previously available. For pediatric patients with high-risk neuroblastoma, Unituxin has provided reduced risk of an adverse event (43% lower) as well as increased overall survival (42% higher) compared to standard 13-cis-retinoic acid chemotherapy. Cholbam, Strensiq, and Kanuma have also provided increased overall survival for patients whose previous standard of care was only symptom-based.
Considerations for Sponsors Seeking a Tropical/Rare Pediatric Disease PRV
As outlined above, PRVs are only granted in special cases in which the drug or biologic application meets certain well-defined criteria. However, Sponsors have no guarantee that the application will be eligible to receive a PRV until the time of approval. Therefore, the best a Sponsor can do is to ensure that their application meets the criteria for receiving a PRV.
To help Sponsors determine eligibility, the FDA encourages early communication with the appropriate review division. Sponsors interested in receiving a PRV should therefore utilize communication pathways such as pre-IND and pre-NDA meetings, as well as communications with the Office of Orphan Products Development to discuss how their application meets these criteria. Furthermore, once the application is ready for submission, the Agency advises that Sponsors include in the original submission a request outlining how the application meets the eligibility criteria for a PRV.
Using all of the available communication pathways with the Agency and including a rationale for receiving a PRV in the drug or biologic submission are the best ways to increase confidence that a PRV will be awarded upon approval of the therapy. For Sponsors determining whether their program might qualify for a rare pediatric disease PRV, the prevalence of the disease in various age groups is of main importance. In certain rare diseases, both children and adults are affected by the rare condition but a PRV is still attainable. In the case of BioMarin’s Vimizim, the indication sought (Morquio A syndrome) is observed in both adults and children, but the FDA determined that the application represented a rare pediatric disease application because the disease in adults is merely a continuum of the pediatric indication. BioMarin’s small clinical program therefore included a combined study with adults and pediatric patients.
When there is uncertainty about the feasibility of obtaining a PRV for a rare condition that affects both children and adults, Sponsors are best served by seeking advice through pre-IND, pre-NDA meetings, and other avenues of communication with the Agency.
Premier Consulting has extensive experience helping Sponsors to communicate effectively with the FDA and to successfully manage their drug development programs. For more information, contact us.