Do you want to leverage FDA’s push to use Model-Informed Drug Development (MIDD*) to accelerate your drug development program?
Premier Consulting’s Pharmacokinetic (PK) experts push the boundaries to reduce drug development programs, which has saved our clients significant time and resources.
Premier’s PK experts use a holistic approach to maximize the impact of MIDD, including:
- In-depth understanding of FDA’s current thinking on MIDD due to frequent interactions with the Agency
- Expertise in exploring different types of PK/PD modeling, with an emphasis on facilitating patient-centric drug development and addressing unmet medical needs
- Using an interdisciplinary team-based approach that leverages Premier’s broad regulatory, scientific, and clinical expertise
Case Studies
The following case studies explore different ways Premier Consulting PK experts have leveraged MIDD to expedite clients’ drug development programs and address critical clinical and regulatory issues for an array of drug types in various therapeutic areas, including small molecules and biologics.
Case Study 1: Optimal Dose Selection while Reducing Clinical Trial Size and Increasing Chance for Success
Objective: Determine a clinically efficacious dose that minimizes Adverse Events (AEs).
Approach: Incorporate clinical PK and pharmacodynamic (PD) data into a PK/PD model to determine the threshold dose where the efficacy endpoint (pain relief) begins to plateau.
Result: PK/PD simulation predicted a plateau in pain relief response at 20 mg.
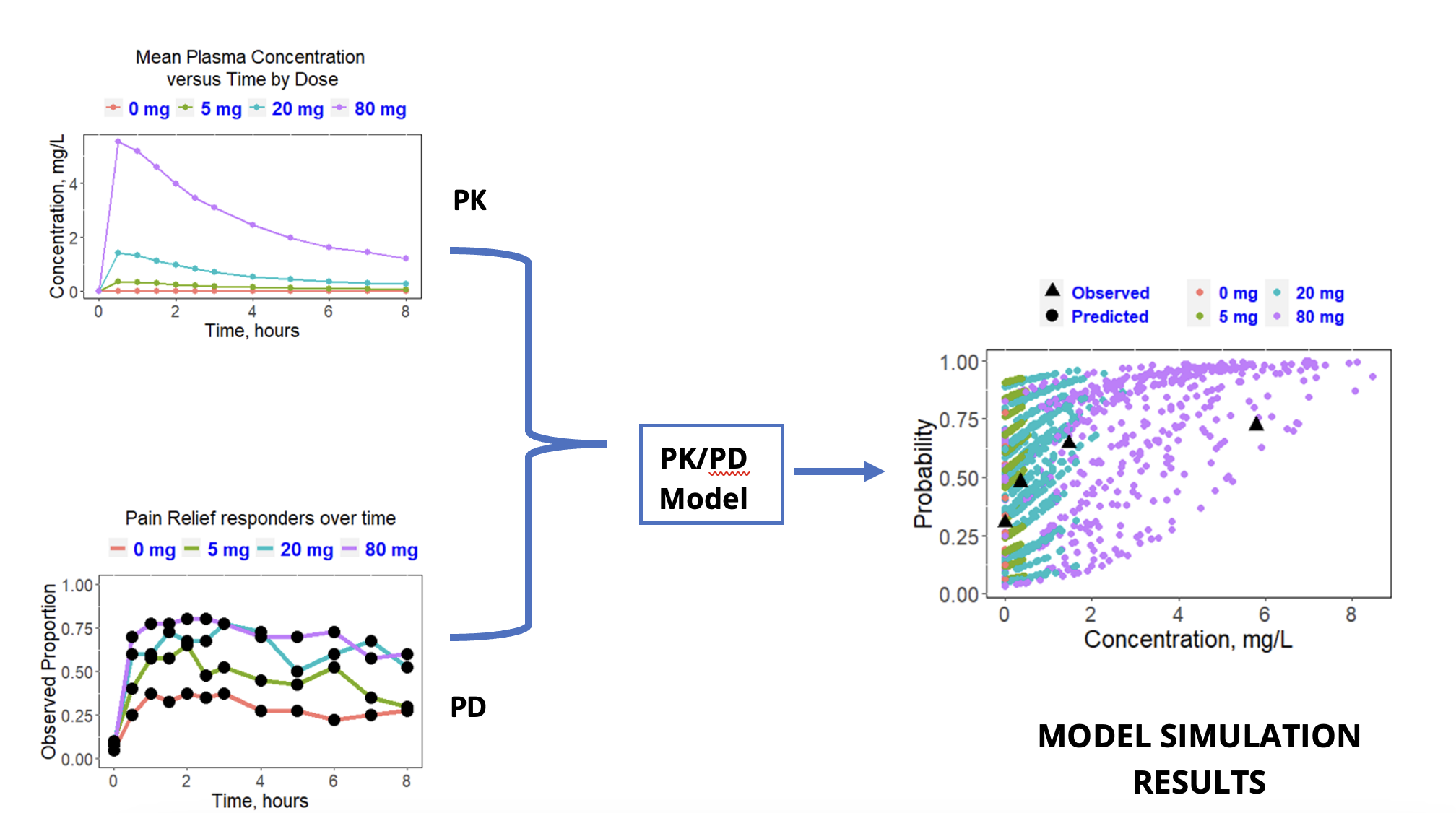
Impact: Recommended a clinical dose to carried forward while reducing the size of a Phase 3 trial and increasing the chances of a successful outcome from both efficacy and safety perspectives.
Case Study 2: Leveraging Data from an Approved Drug to Eliminate Clinical Studies for a New Product
Objective: Demonstrate the same efficacy for two drugs (one approved and one new) with similar exposure but different PK profile curves.
Approach: The efficacy of the new drug was evaluated by the ability to stabilize a PD biomarker. PK data was used to model the profile of the PD marker to assess efficacy using exposure-response modelling.
Result: The maximum PD marker levels, Cmin concentration and Area Under the Effect Curve (AUEC) were comparable between the new drug and the approved drug.
Impact: No additional bridging study was required, which allowed safety data from the approved drug to be used to support approval of the new drug and reduce the size of the clinical program.
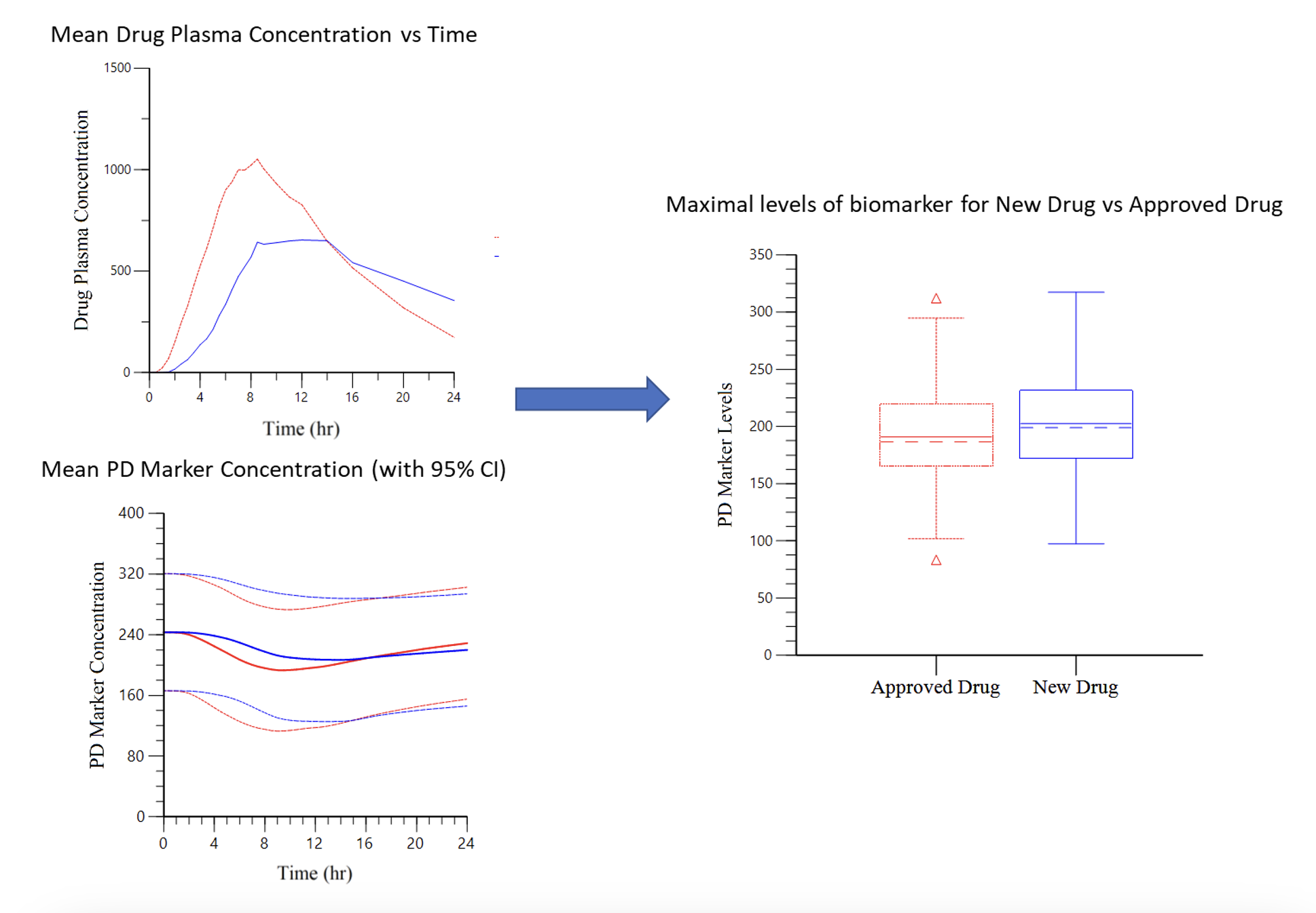
Case Study 3: Improving Clinical Trial Success Through Clinical Dose Modeling
Objective: Estimate detectable exposure levels for a topical cream by leveraging PK data from an oral drug with the same active pharmaceutical ingredient (API).
Approach: Use available PK parameters from the oral drug to simulate oral PK profiles and then convert to topical exposures based on bioavailability (BA).
Result: Modeling the topical dose with percent Body Surface Area (%BSA) coverage predicted quantifiable concentrations and resulting efficacy.
Impact: Clinical dose setting was improved and increased the chance of a successful study by meeting the clinical endpoint.
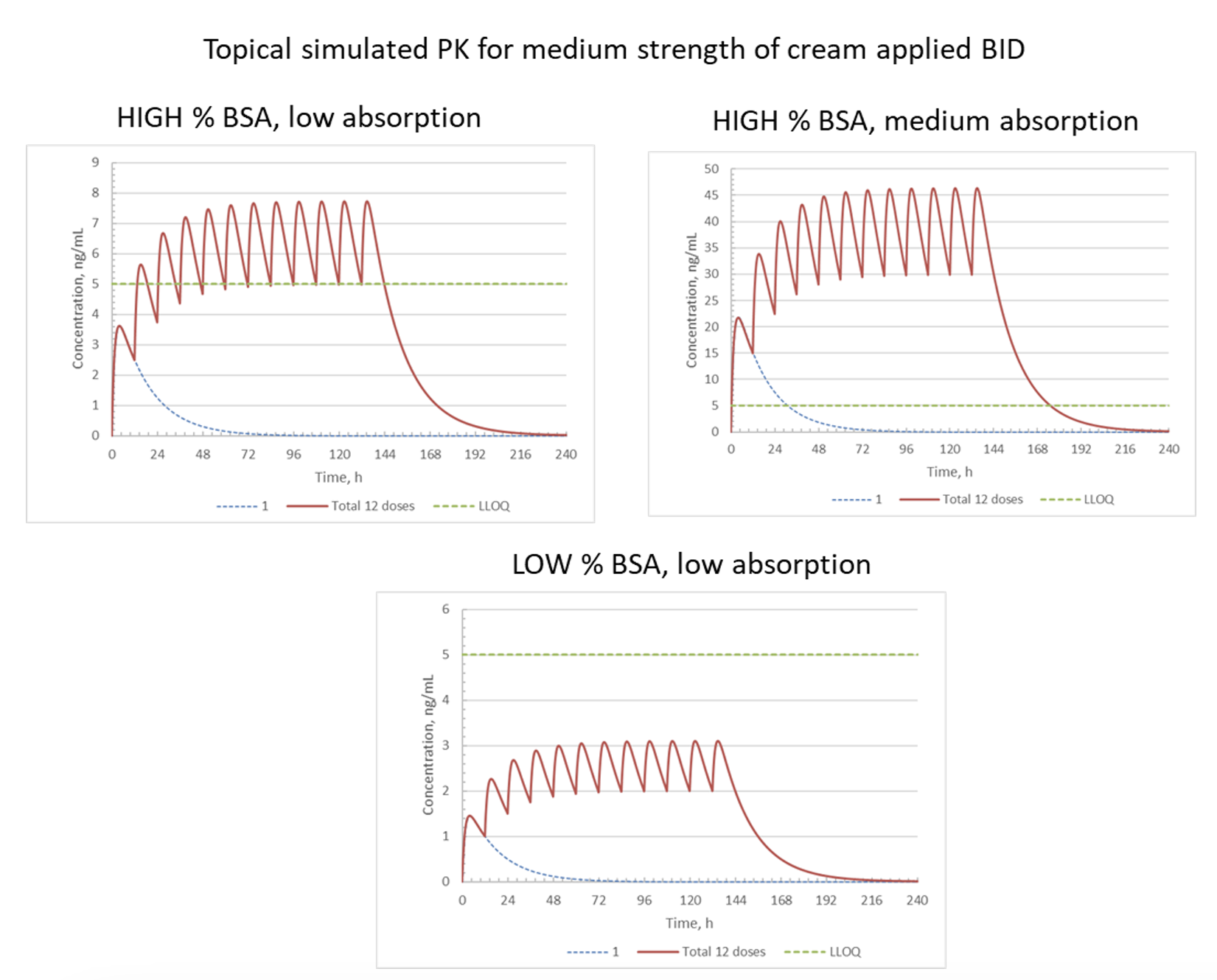
NOTES: %BSA = relative surface area of skin where topical cream is applied; BID = twice daily; dotted blue line = PK profile after single dose; red line = PK profile after each of 12 BID doses; LLOQ = lower limit of quantitation
Case Study 4: Optimizing Clinical Efficacy Through Alternative Route PK Modeling
Objective: Determine the drug administration route with the best efficacy
Approach: Population PK/PD modeling to determine the lower and upper concentration thresholds and clinical effect for different routes of administration, dose levels, and patient health conditions.
Result: Model demonstrated differential exposure for intravenous (IV), oral, and subcutaneous (SC) routes of administration. SC administration was determined to provide the most favorable exposure profile that maximized efficacy under the widest range of conditions.
Impact: A direct comparison was made for different doses administered via various routes and conditions. The results guided a commercial assessment and selection of the optimal dose, dosage form and route.
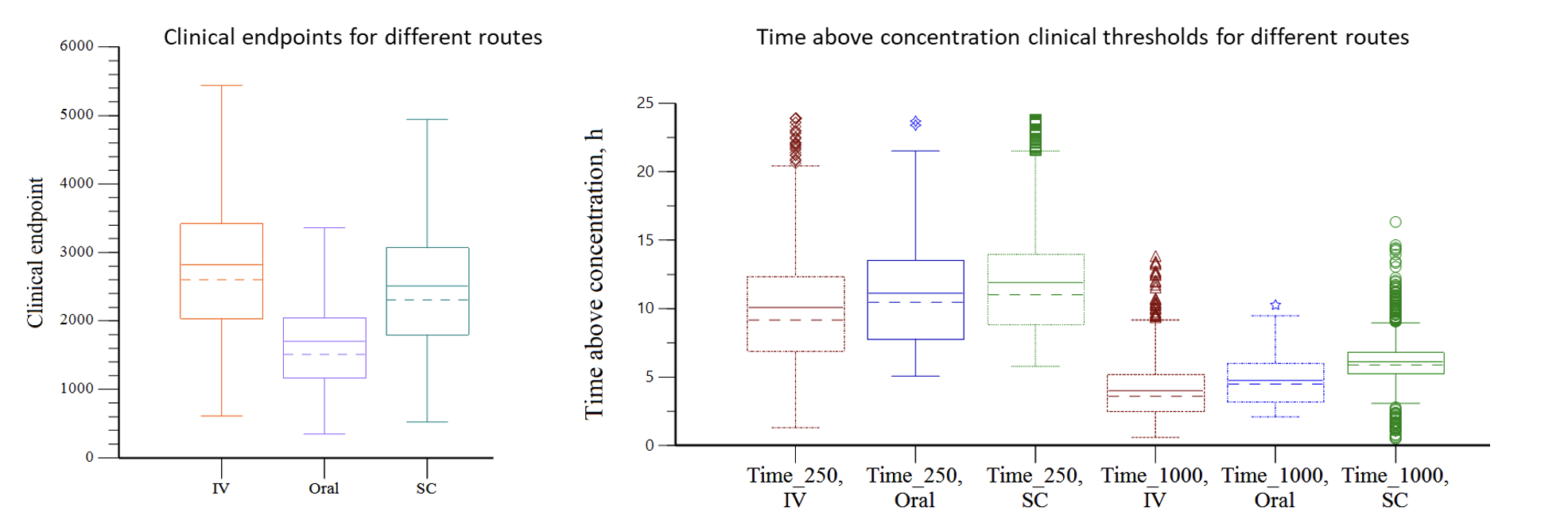
NOTES: Time_250 = time above concentration of 250 units, Time_1000 = time above concentration of 1000 units