Let’s Move Your Program Forward. Together.
Wherever you are on your development journey, Premier Consulting’s cross-functional team of experts can help you reach your next milestone.
Wherever you are on your development journey, Premier Consulting’s cross-functional team of experts can help you reach your next milestone.
We are scientists, researchers, strategists, and innovators on a mission to help biotech innovators transform their life-changing ideas and breakthrough science into new medical treatments, devices, and diagnostics.
Premier Consulting provides strategic product development and global regulatory consulting services with unparalleled nonclinical, CMC, quality, clinical, and commercial expertise. We offer end-to-end strategic insights to help biotech companies advance their programs through every development milestone, from the earliest stages of strategy and planning, through regulatory approval, to commercialization.
Across the development lifecycle, our integrated solutions and cross-functional team provide customized support for your program. Providing a full range of strategic support, we help sponsors navigate their programs confidently and gain regulatory approval in the most cost- and time-efficient way possible.
We offer a full range of regulatory support services, from product concept to submissions to regulatory interactions. We thoroughly analyze the issues associated with your unique program and assess the regulatory environment, acting as either an extension of your in-house regulatory team or your full-service regulatory department depending on your needs. With our help, you can approach regulators with confidence — minimizing risk and negotiating from a position of knowledge and strength.
By delivering integrated nonclinical strategies, we get you to the clinic faster and support your continued clinical development. When you partner with Premier Consulting for your nonclinical program, you can reach critical development milestones on time and within budget.
Since early planning in product development can save you significant time and money, our experts advise on everything from formulation to site inspections to protocol design from the earliest stages of your program. We have managed numerous successful CMC dossier submissions for clinical trial and marketing applications, and our network of CMOs allows us to identify the most suitable service providers for you.
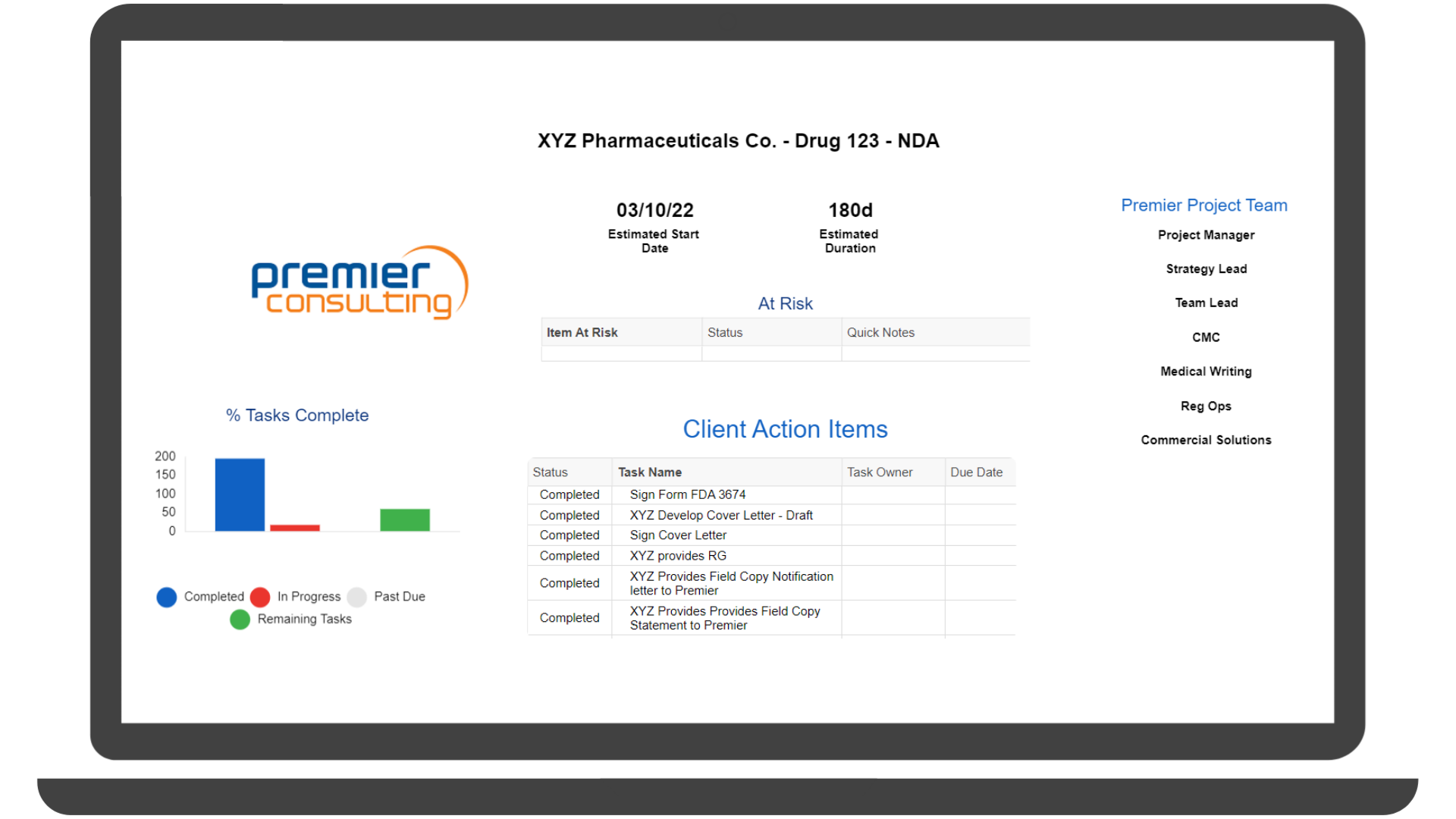
We prepare programs for success from concept to market, serving as the single point of contact through the entire regulatory project lifecycle. Our project managers strategically position and guide clients through a streamlined and customized approach that helps them achieve their drug development goals, leveraging elevated tools such as SmartSheet timelines and Dashboards.
Contact us to find out how we can support your program!
We provide medical writing and editing services for a wide variety of regulatory documents, ranging from health authority interaction submissions through post-marketing commitments. Familiar with regional healthcare authority requirements, ICH and GCP guidelines, and best industry practices, our writers have extensive experience supporting both single-document and large-scale submissions in eCTD format.
We identify crucial clinical pharmacology needs and deliver integrated clinical pharmacology, pharmacokinetic, and bridging strategies to move you efficiently through clinical development, while avoiding unnecessary and costly studies. Partner with us for your clinical pharmacology program to streamline overall development and meet critical milestones.
To discover how these and other Premier Consulting solutions can advance your drug, biologic, or device to the next phase of development, contact us today!