Premier Partners
Success Starts with the Right Partners
For even the most experienced drug development teams, choosing the right strategic partners is crucial. Program success is at stake, and you need a team of experts you can trust to validate the safety and efficacy of your molecule. We can help.
Experts in Nonclinical Study Design, Monitoring, & Logistics
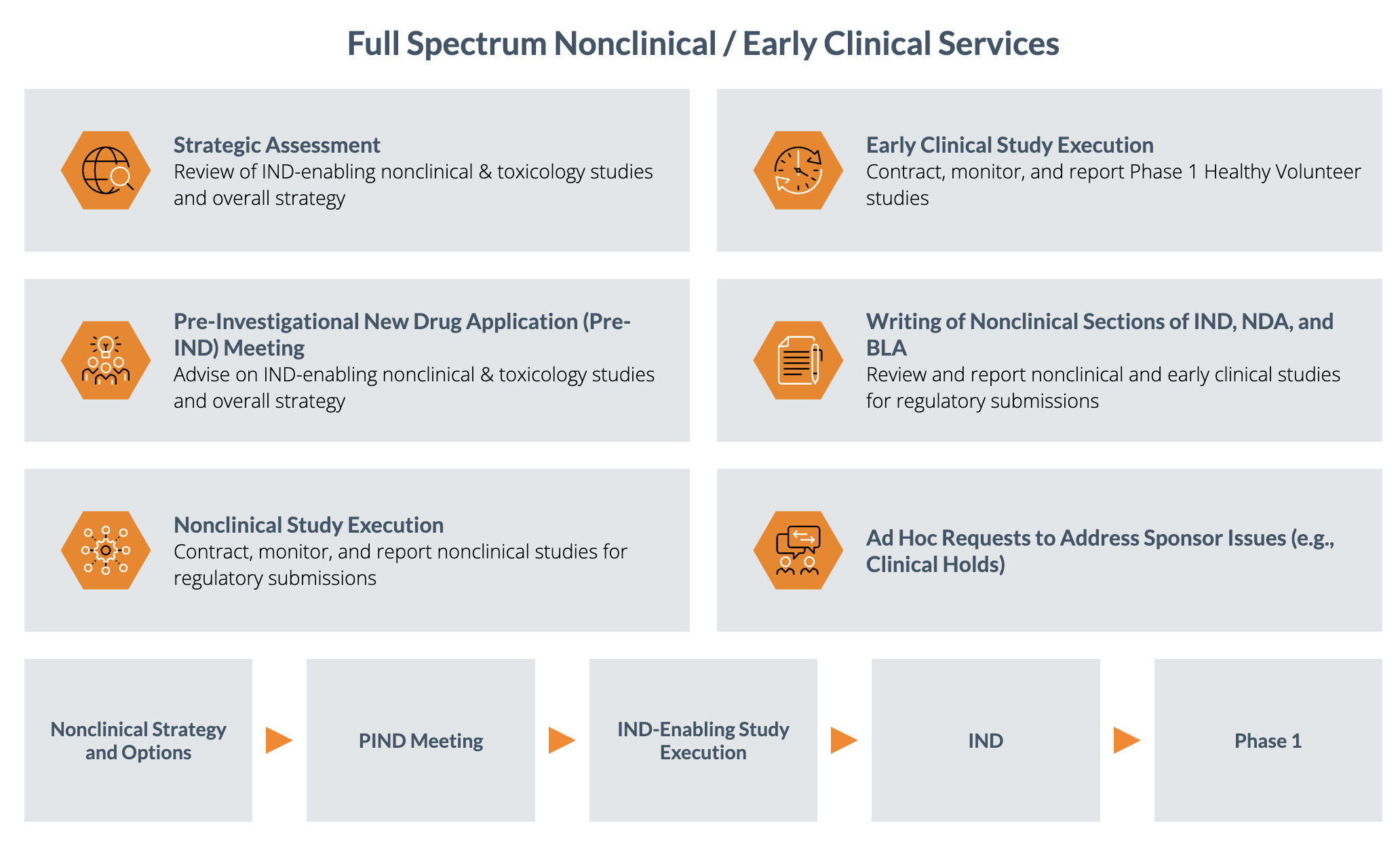
Premier Partners is a network of independent drug development experts that provide high-touch and cost-effective nonclinical and early clinical support, including study design, monitoring, and logistics. The Partners deliver comprehensive early development services, backed by Premier Consulting’s cross-functional expertise – to bring all aspects of development together and offer a seamless transition to your next milestone.
Meet the Partners
Our experienced teams, combined with the cost advantages built into our operating model, position us to meet the needs of small to mid-size biotechs. We are dedicated to providing customized solutions for the entire duration of your program:
- Program review, gap analysis, and study planning
- Study design and optimization
- Laboratory procurement and auditing
- Program oversight
- Vendor and logistics management
- On-site study monitoring, included at no additional cost
- Study data analysis and reporting
- Regulatory consulting
- Cost-saving R&D in Canada

Experience
Our team brings diverse scientific and product development expertise in areas including ADME/PK, bioanalysis, CMC, pharmacology, toxicology, and early clinical development. We collaborate with Premier Consulting’s Development Strategists to streamline and advance drug programs, while leveraging Premier Consulting’s Quality Management support capabilities to stay at the forefront of quality and compliance standards. The compounds and studies we support include: