Shortening the Review Clock: the Latest on Priority Review Vouchers
The Latest on Priority Review Vouchers
Priority review vouchers (PRVs) are a valuable incentive available to companies upon approval of novel drugs to treat rare pediatric diseases or certain tropical diseases. These vouchers can be used on a subsequent application to speed the review process and are an asset that can be sold or transferred an unlimited number of times to other companies. The advantage of the shortened review clock could allow a voucher holder’s product to be reviewed and approved ahead of a similar competing product. The shorter review time also provides a mechanism of faster access to drugs for patients with rare or tropical diseases.
Previous blog posts on this topic can be found here and here. Last year, the FDA released a guidance on tropical disease priority review vouchers that details the criteria for obtaining a voucher and the parameters for use of the voucher.
The purpose of this program, established in 2007 (for tropical diseases) and 2012 (for rare pediatric diseases), was to incentivize the development of new therapies for certain diseases. In our 2015 blog post on these vouchers, we detailed the rules for classifying a drug to treat a tropical or rare pediatric disease. Briefly, for a drug to qualify for the PRV program, it must:
- Be for the prevention or treatment of one of 19 tropical diseases or a rare pediatric disease
- Not contain any active ingredient (including any ester or salt of the active ingredient) that has been approved in any other application
- Qualify for priority review (i.e., offer a major advance in treatment or provide treatment where no adequate therapy exists)
The application must be submitted under section 505(b)(1) of the FD&C Act, which includes 505(b)(2) applications, or section 351 of the PHS Act. Qualifying applications will be issued a PRV at the time of marketing approval.
As discussed in a previous blog post, the rare pediatric disease PRV program was set to expire at the end of 2016. However, the 21st Century Cures Act signed into law in December 2016 included an extension of the program to 2020*. There are no restrictions on the tropical disease PRV program in terms of the number that can be granted or a program expiration date.
Acquisition and Fate of Priority Review Vouchers to Date
At the time of our previous blog post in July 2016, 9 total PRVs had been granted (including 2 granted under the 505(b)(2) pathway in 2015). Four additional PRVs, three for rare pediatric diseases and one for a vaccination against a tropical disease, have been granted, as shown in Table 1. One rare pediatric disease voucher was granted for a product (Emflaza) approved by the 505(b)(2) pathway.
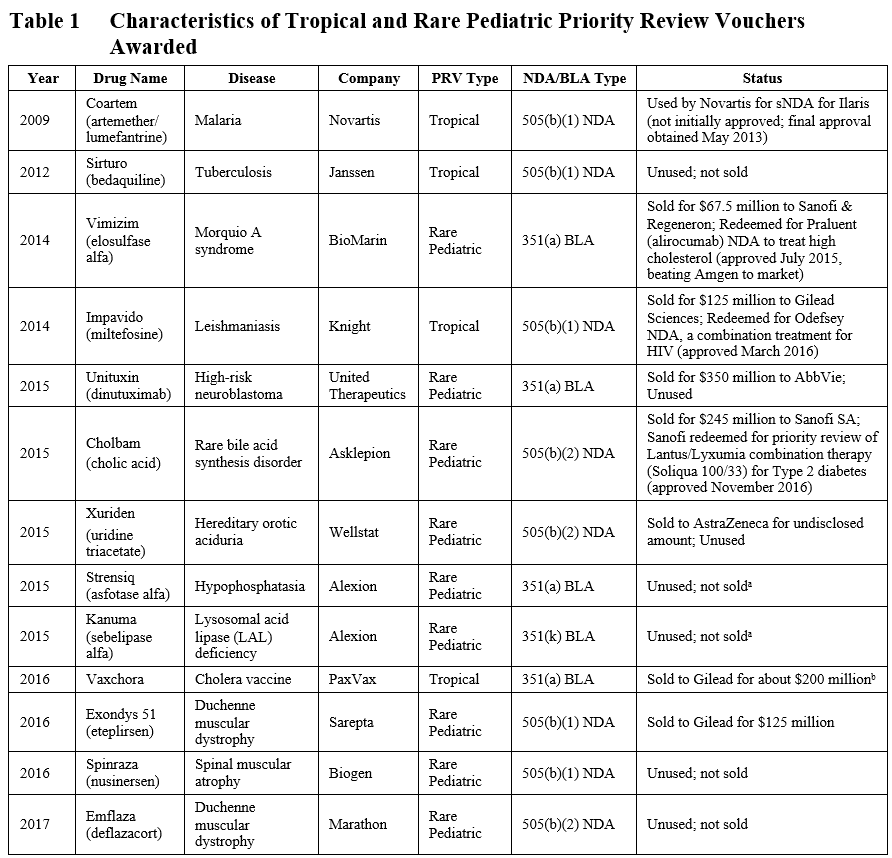
a Alexion has noted they would like to keep one of their PRVs.
b Actual amount not disclosed; amount of ~$200 million was calculated based on disclosures of 2016 R&D spend (see here).
Thus far, two of these recently granted vouchers have sold – PaxVax’s tropical disease voucher sold to Gilead for approximately $200 million and Sarepta’s rare pediatric disease voucher sold to Gilead for $125 million (see Table 1). The highest grossing PRVs sold to AbbVie for $350 million and Sanofi for $245 million in 2015.
Several changes could influence the scarcity value of PRVs, and therefore, the price.
- First, in 2015 there was a limit to the issuance pediatric PRVs set to end in March 2016; this has since been extended (initially through 2016 and currently to 2020*).
- Further, 8 PRVs have not been used (5 have not been purchased and 3 have been purchased but not used and therefore could potentially be sold again). Initially there was a limit on the transferability of the tropical disease vouchers; each could only be sold once. This limit has been removed, so these vouchers can be sold and resold until they are used, similar to the pediatric PRVs.
- Additionally, there is no end date for the issuance of tropical disease vouchers (unlike the pediatric PRVs).
- Finally, price paid is determined not only by supply, but also demand. To provide sufficient value, a PRV will need to be less than the potential extra 4 months of profits of the drug, requiring blockbuster initial sales or a situation like that of Sanofi and Regeneron utilizing a PRV to expedite the review process ahead of a competitor product.
Considerations for Sponsors Seeking a PRV
The extension of the expiration date for granting rare pediatric disease vouchers removes some of the uncertainty in their immediate availability to sponsors of these programs. However, PRVs are granted only in specific situations in which a drug or biologic application meets certain criteria (outlined above).
Although sponsors may seek a rare pediatric disease designation, the voucher is not awarded until approval of the marketing application (NDA for drugs, BLA for biologics) based upon eligibility criteria at that time. While the designation does not have to be sought in advance**, early communication with the Agency can be useful in terms of determination of eligibility.
Premier Consulting has extensive experience helping sponsors communicate effectively with the FDA to successfully manage and develop effective strategy for drug development programs. For more information, contact us.
*Section 3013 of the 21st Century Cures Act allows a drug that is designated as a drug for a rare pediatric disease prior to September 30, 2020, to receive a priority review voucher upon approval of an NDA or BLA through September 20, 2022. This is an extension from the previous expiration date of December 31, 2016.
**Although designation is not required for a PRV to be granted at the time of approval, sponsors intending to obtain a PRV upon approval after September 30, 2020 should apply for the designation per Section 3013 of the 21st Century Cures Act.