Orphan Drug Development: Ensuring Best Time to Market
The Benefits of 505(b)(2) for Orphan Drug Development
The Orphan Drug Designation Program, created by the Orphan Drug Act of 1983, provides significant financial incentives for the development of drugs for rare diseases. The potential incentives include tax credits for clinical trial costs, waiver of the Prescription Drug User Fee for NDA filing (~$2.4 million in 2016), and 7 years of market exclusivity. To qualify for these incentives, a drug developer must apply for orphan designation from the FDA’s Office of Orphan Products Development (OOPD).
Due to the ever increasing popularity of the program, the OOPD has recently adjusted its review timeline target from within 90 days of receipt to within 120 days of receipt. The OOPD stated that, on average, a designation request goes through 2 review cycles; taken together with the time to prepare (and revise) the request itself, the process could take a year or more, longer than the NDA review itself. For a smaller drug development program, as often seen in 505(b)(2) programs, a year can cover a significant portion of your development timeline.
Orphan Drugs and 505(b)(2) by the Numbers
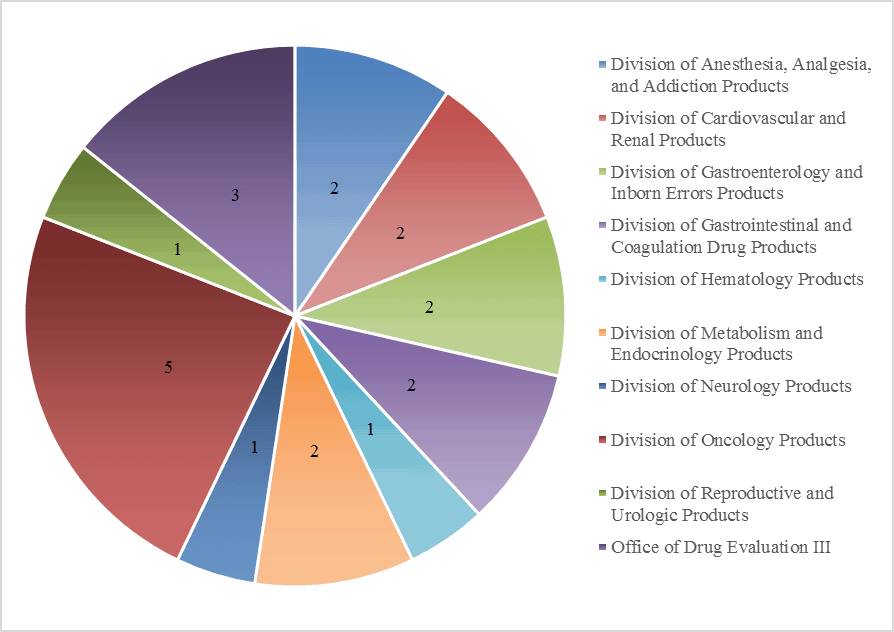
At Premier Consulting, we analyze the paths to approval for the orphan drug products that have been approved via the 505(b)(2) regulatory pathway. Through our proprietary research on 505(b)(2) approvals, we have identified 21 approved 505(b)(2) products from 2003 to 2015 (19 with available SBAs). The products vary by indication and were approved by 9 different review divisions (Figure 1). Nineteen of the 21 products are designated reference listed drugs in the FDA Orange Book. Dosage forms vary, but generally utilize oral or injectable routes of administration.
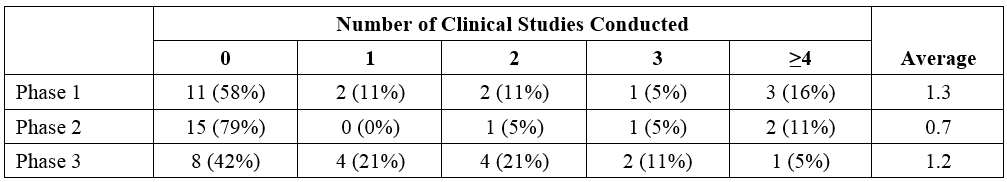
The average number of clinical studies (Phase 1, Phase 2, and/or Phase 3) conducted per product (19 with information available) was 3.1 (Table 1).
- For 6 of the orphan products, no clinical studies were conducted;
- Of these, 3 relied on literature and 1 relied on a biowaiver.
- Notably, 3 of these products were indicated for acute poisoning events (cesium or thallium; plutonium, americium, or curium; and cyanide).
- One orphan product was approved with a bioequivalence program consisting of two Phase 1 studies only.
- One product was approved with only Phase 2 studies and four products were approved with only Phase 3 studies.
- Of the 19 development programs, 11 conducted nonclinical studies;
- 5 of the products had extensive nonclinical programs (inclusive of pharmacodynamics, pharmacokinetic, and toxicity studies).
For products containing a new molecular entity or new active ingredient (n = 9), the average number of clinical studies conducted was 4.7 (1.7 were Phase 3 studies); for products containing a new dosage form or new formulation (n = 10), the average number of clinical studies conducted was 1.7 clinical studies (0.7 Phase 3 studies).
Benefits of Orphan Drug Development
An added benefit of developing an orphan drug is that they often qualify for priority review, a designation that is applied to drugs that would be significant improvements in the safety or effectiveness of treatment, diagnosis, or prevention of serious conditions when compared to standard applications. While the standard NDA review time is 10 months, the review goal is 6 months for priority drugs.
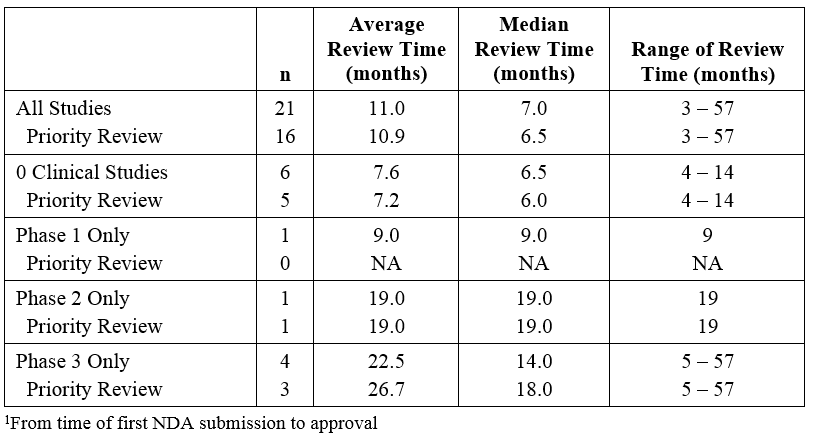
Premier Consulting evaluated the effect of receiving a priority review designation on the length of the NDA review time for orphan 505(b)(2) products (Table 2). Sixteen (16) of the 21 products received priority review, but the average review time was similar to that of products that did not receive the designation. This did not seem to be affected by the clinical development program conducted (no studies, Phase 3 only, etc.).
Orphan Drug Development Experience
Taken together, Premier Consulting’s proprietary analysis of orphan drug development programs shows that the development programs for these drugs can progress very rapidly, and utilizing the 505(b)(2) regulatory pathway can further streamline the development timeline. Premier Consulting recognizes that time saved in any drug development program can be rewarding for the Sponsor.
Premier Consulting is the industry leader in 505(b)(2), with involvement in 26 orphan drug approvals and 8 more currently in process. Premier Consulting has extensive experience in preparing Orphan Drug Designation requests and can ensure you don’t lose any time developing your orphan drug.