Nonclinical Development Strategy and Study Design
With drug discovery completed and your lead asset identified, it’s time to initiate IND-enabling activities, moving one step closer to clinical deployment.
According to the Tufts Center for the Study of Drug Development, fewer than 10 percent of nonclinical lead assets move into the clinic. The design, development, and delivery of your nonclinical program play a critical role in whether your program advances to Phase 1 and how long it takes to get there.
As you set out to gather the data to submit your IND application, the stakes are high, and not all variables are within your control. Getting it right with the variables you do control avoids exposing your program to unnecessary risk and gives your product the best chance to reach the clinic.
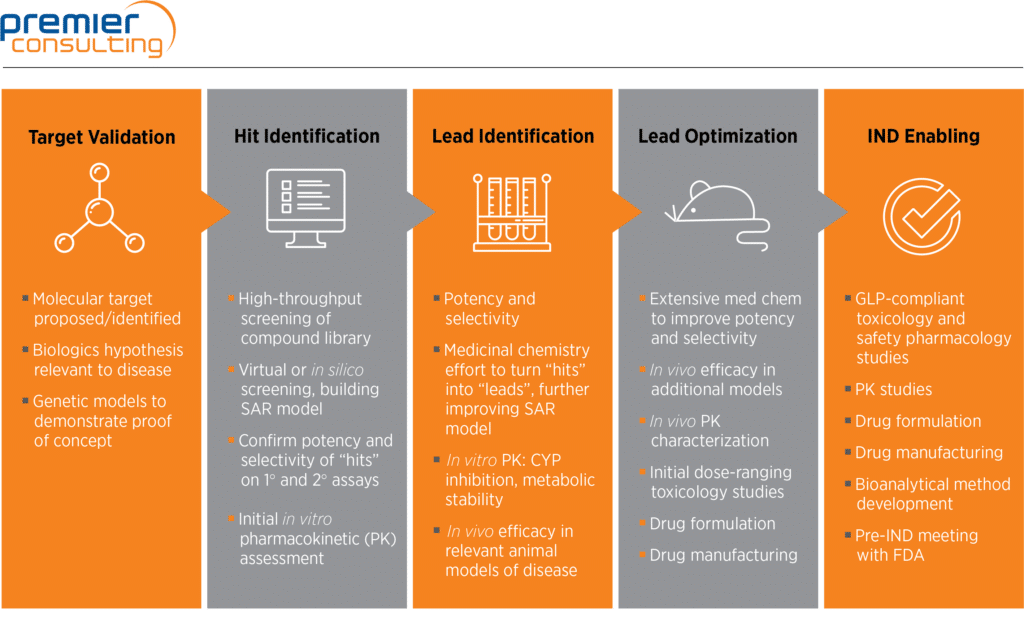
This chart is intended to provide an overview of nonclinical development activities. Required studies and IND-enabling activities are determined based on product characteristics, indication, and approval pathway. Premier Consulting specializes in evaluating products and designing strategic development programs that define the most aggressive, optimized path to approval. Contact us to learn more.
Nonclinical strategy and study design
Animal safety pharmacology, pharmacokinetic, and toxicology studies provide nonclinical data that allow regulators to assess whether your product is reasonably safe for initial testing in humans. Successful nonclinical strategies leverage smart design and planning to identify the right studies and outline an optimized plan to de-risk critical early-stage milestones.
It’s important to note that the transition into the clinic is most successful when the right manufacturing partners are involved early and often in nonclinical strategy discussions. Determining quality and compliance standards for product development and manufacturing scale-up early helps avoid roadblocks later in development.
Selecting the right studies
In the race to the clinic, high-volume, low-cost nonclinical studies are enticing: Quickly conduct safety pharmacology, pharmacokinetic, and toxicology studies to set the starting dose for the clinic and conserve cash flow for the next phase. But what is the cost of running a study you didn’t really need in the first place? Or worse, a study that yields data regulators won’t accept?
As you consider which nonclinical studies are necessary for your program, assemble the right experts to determine the best strategy based on your product and the intended indication. Studies may include:
- Primary pharmacology (animal models of efficacy)
- Secondary pharmacology (off-target effects)
- Safety pharmacology
- Pharmacokinetics, including ADME (absorption, distribution, metabolism, and elimination)
- General toxicology
- Genetic, carcinogenicity, and repro/developmental toxicology
- Special studies (e.g., phototoxicity, juvenile toxicity, combination product, drug abuse liability, impurity/degradant qualification)
Prioritizing studies to de-risk your program
While it’s likely that most of the studies listed above will be required as part of your nonclinical program, not all are necessary to open an IND application. Regulatory expertise and a clear understanding of known safety and efficacy risk factors are key to prioritizing studies to de-risk your program. It can save you from investing time and money in a product that may not achieve your development goals.
For all development programs, we recommend range-finding studies to assess safety based on different routes of administration, formulations, or dosing regimens. Even programs that might rely on existing literature for safety information can benefit from range-finding studies. Recently, a client seeking approval via the 505(b)(2) regulatory pathway conducted a range-finding study in two species. The literature and study results did not line up. Had the client advanced directly into pivotal GLP studies, it’s likely that the safety concerns addressed through the study would have presented major roadblocks, including avoidable delays and expenses, later in development.
Designing with GLP in mind
For certain nonclinical studies (e.g., pivotal safety pharmacology and toxicology studies, both in vitro and in vivo), compliance with good laboratory practices (GLPs) is required. The goal of these requirements is to ensure the quality and integrity of the safety data collected.
One common mistake is species selection based on initial purchase price. Toxicology studies should be based on the known pharmacology and toxicology of your drug to provide greater understanding of potential toxicity. The FDA typically expects “relevant species” be used in the nonclinical program. A variety of in vitro and in vivo techniques, assays, and functional tests can be used to identify relevant species, including target profiling, expression of appropriate and responsive drug target in animals, and similar local anatomy or tissue structure for area of drug administration.
Beyond species selection, there are several other important areas to consider as you design GLP-compliant studies:
|
Study Design |
Considerations |
| Animal procurement | – Regulatory requirements for selection of species – CRO experience with model – Animal-human correlation of model |
| Health | – Breeder quality and history – In-house checks upon receipt – Shipment recovery and acclimation |
| Housing | – Cage and bedding type – Macro- and microenvironment – Single vs. social co-housing |
| Identification | – Animal ID (e.g., RFID, tag, tattoo, collar, punch) – Identification verification (e.g., barcoding) |
| Environmental | – Temperature and humidity – Light/dark cycling – Ventilation (HEPA) and pressure (positive and negative) – Food and water quality and assessments |
| Randomization | – Weight distribution – Health status |
| Dosing | – Same time of day and duration – Route of administration effects (e.g., oral, dermal, inhalation) – Staggered start to accommodate downstream work (e.g., necropsies) |
| In-life | – Acclimation period physical exam – Clinical observations – Body weight and food monitoring – Ocular examination, preferably by a board-certified veterinary ophthalmologist – Electrocardiogram in large animals and assessment by a board-certified veterinary cardiologist – Clinical pathology |
| Termination | – Selection of a board-certified veterinary pathologist – In-life data review by pathologist – Necropsy – Histopathology |
Moving toward IND filing for your lead candidate can be a lengthy and costly process. Developing the right strategy and study designs is the first step in the right direction toward an optimized nonclinical program. Whether you need specialized expertise to design optimized nonclinical toxicology studies or a fully integrated nonclinical development team to serve as an extension of your virtual team, Premier Consulting offers a wide range of customized nonclinical solutions to support your early development needs. Contact us and let’s discuss how we can support your program.