Key Questions You Must Ask Before Hiring a Drug Development Consultant
Updated on: March 9, 2021
Emerging biopharma companies play a big role in overall drug development—the percentage of new therapies both originated and launched by these companies has increased over the last decade, in an industry that is growing at the same time. As drivers of innovation, emerging biopharma companies face different challenges from mid-tier and large pharma, especially in the earliest stages.
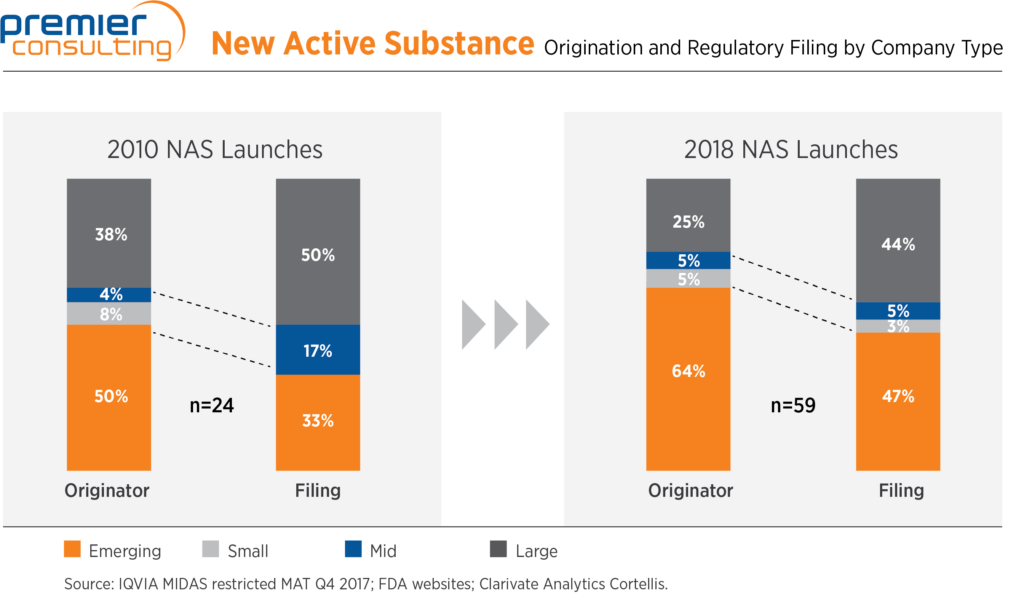
Most prominently, while most have deep experience in their therapeutic or scientific areas, their teams often lack a sufficient breadth and depth of expertise in other necessary disciplines. As a result, consultants play a significant role in mapping out and executing their product development plans.
Extending the expertise of a management team through consultants can have a huge impact on the success of a company in raising capital and developing a product, but emerging biopharma companies often have very little experience in hiring outside consultants and knitting them together with their own teams to drive effective collaboration. Drug development consultants promise many things, and the right consultants can make a large difference in the speed and success of development, perhaps even the difference between overall success and failure. Sponsors should ask several key questions before hiring a drug development consultant:
- Does the consultant have the expertise to de-risk my program and enhance the probability of success?
- How does the consultant define success?
- Will the consultant be an effective collaborator with my management team and other consultants?
Does the consultant have the expertise to de-risk my program?
Expertise requires both technical knowledge in a discipline and relevant experience in similar or analogous situations. One without the other is not enough. Think of a white water raft moving down a choppy river: The river guide must have both the technical skill to steer and manage the boat and previous experience traveling the river, to know what is coming around the next turn and lead the boat successfully through.
Management teams know the knowledge and experience gaps they need to round out through consultants. All consultants will claim to be experts at what they do, so each biopharma company must evaluate a consultant’s knowledge and the experience to discover if its expertise lines up with the company’s needs. Regardless of a potential consultant’s discipline, consider the following when evaluating its fit:
Scientific & Medical Experience
Does the consultant have knowledge and experience in the scientific and therapeutic area? Does it understand the full potential of the program, and can it help select the right unmet medical need, target patient population, etc.? Does it appreciate factors such as rare disease patient populations and other unique elements that influence development?
FDA/Regulatory Experience
Working with the FDA is as much an art form as it is a matter of following procedure. The FDA will not prevent a sponsor from doing more than the minimum if the sponsor proposes a development plan that is larger than necessary. Does the consultant have experience with the FDA or other regulatory authorities? Can it anticipate FDA guidance? Can it approach development planning with the creativity needed to optimize time and cost to approval?
Integration of Disciplines
Some decisions make sense in isolation but put extra strain on the overall program cost and timeline. Does the consultant understand how its activities integrate with the other scientific, commercial, medical, and regulatory demands of the program?
Global Considerations
Understanding a product’s global potential may impact important development decisions. Does the consultant have experience with multiple markets and understand how to incorporate global considerations into product development plans?
How does the consultant define success?
Success goes beyond completing a scope of work or a project. It is also bigger than simply getting a drug approved. The best consultants are ones that look at the “big picture,” that understand the tradeoffs a company must consider in setting priorities and how those tradeoffs might adjust the course of action within their specific disciplines. They can look beyond the most efficient approval path to consider the commercial viability of the product five and 10 years down the road.
Will the consultant be an effective collaborator?
Managing multiple consultants for a given project can be challenging, as communication or collaboration barriers between different groups can hinder a project and its timeline. Even if a contract is narrow and brief, a consultant becomes part of a sponsor’s team. The consultant should work well with the team, be invested in the product, and be willing to take on some of the risk.
A consultant should strive to understand the key elements of a program and work with the sponsor to identify potential challenges and roadblocks that might not have been previously identified. While most consultants can help guide a sponsor through the basics, it is best to have a partner that is not afraid to push back on potential weak areas of a program or to ask tough questions, because the FDA will most likely ask the same questions during the approval process. A good consultant is not afraid to say what a sponsor needs to hear, even if it means less potential business for either party.
It is also important for consultants or consulting teams to be effective communicators. Do they listen well? Are they open to feedback and constructive criticism? Are they team-oriented, or do they prefer siloed interactions? While the answers to these questions can be hard to discern in evaluation, management teams should be intentional in assessing a consultant’s effectiveness as a collaborator before signing on the dotted line.
Premier Consulting’s Unique Approach to Drug Development
Premier Consulting is a strategic drug development company that takes a creative approach to address the unique needs of our clients. A full, multidisciplinary team of PhD scientists and specialists is dedicated to each project, and we approach each challenge as a fresh opportunity to discover the most creative and efficient way to facilitate a product’s market success.
We become part of your team, working together with frequent communication and clearly defined goals. Our project teams include regulatory, toxicology, CMC, and other expertise to bring a holistic approach to programs, and each project is fully customizable based on your project goals and established capabilities. Premier Consulting has supported hundreds of programs in all disciplines to drive successful outcomes.
We look forward to learning more about your project and goals and to determining ways we can help you to meet them. Contact us to learn more.